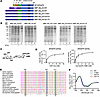
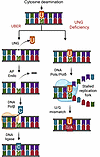
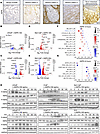
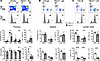Hair follicle epithelial stem cells contribute to interfollicular epidermis during homeostasis
Ghotbi et al. demonstrate that transcription factor KROX20 marks a stem cell population in the hair follicle whose lineages contribute to the interfollicular epidermis. In the cover image, the green spheres within the upper hair follicle region represent KROX20-positive stem cells that migrate up to maintain proper epidermal homeostasis. Image credit: Elnaz Ghotbi, generated using ChatGPT.
In-Press Preview - More
Abstract
Authors
Jayanta Mondal, Patrick Nylund, Prit Benny Malgulwar, William E. Johnson, Jason T. Huse
Abstract
We conceived of a type of antitumor mechanism of action by which a soluble target in the tumor microenvironment, such as a tumor-driving growth factor, can be phagocytized along with cancer cells via antibody-dependent cellular phagocytosis (ADCP) using an antibody bispecific for the soluble target and a solid target overexpressed on the cancer cell surface. We explored this concept through engineering bispecific antibodies (BsAbs) co-targeting human epidermal growth factor receptor-2 (HER2) and vascular endothelial growth factor A (VEGFA) in an scFv-IgG format (VHS). We showed that the HER2-VEGFA BsAbs but not the parental antibodies alone or in combination induced co-phagocytosis of VEGFA and HER2-overexpressing cancer cells by tumor-associated macrophages via ADCP. In both immunocompromised and immunocompetent mice with aggressive tumors, the BsAbs demonstrated greater anti-metastasis activity and produced a greater survival benefit than the parental antibodies alone or in combination, in a manner dependent on Fcγ receptors on the macrophages. Our results provide proof of the concept that HER2-VEGFA BsAbs achieve enhanced antitumor activity by leveraging HER2 overexpressed on the cancer cell surface to induce co-phagocytosis of VEGFA. Our findings warrant clinical testing of the strategy to treat metastasis and recurrence of HER2-overexpressing solid tumors that respond to anti-VEGFA therapy.
Authors
Yang Lu, Songbo Qiu, Zhen Fan
Abstract
BACKGROUND. Understanding age-associated differences in acute and memory adaptive immunity to SARS-CoV-2 and how this contributes to more favorable outcomes in children is critically important. METHODS. We evaluated SARS-CoV-2–specific T cell, B cell, and antibody responses in 329 peripheral blood samples collected from non-hospitalized children, adolescents, and adults at three timepoints, including acute and memory timepoints. RESULTS. Most children produced robust CD4+ T cell responses during infection and developed memory CD4+ T cells; however, young children <4 years old often had undetectable CD4+ T cell responses compared to older children and adults. Young children also generated reduced frequencies of memory B cells; despite this, they mounted substantial and durable neutralizing antibody responses. CD4+ T cell responses in children were biased towards non-spike epitopes, especially in asymptomatic cases. Memory B cells in children were preferentially classical memory or, paradoxically, CXCR3+. CONCLUSION. These findings support the concept that the kinetics and composition of T and B cell responses shift across age groups and may be associated with milder COVID-19 outcomes in children.
Authors
L. Benjamin Hills, Numana Bhat, Jillian H. Hurst, Amber Myers, Thomas W. Burke, Micah T. McClain, Elizabeth Petzold, Alexandre T. Rotta, Nicholas A. Turner, Alba Grifoni, Daniela Weiskopf, Yvonne Dogariu, Genevieve G. Fouda, Sallie R. Permar, Alessandro Sette, Christopher W. Woods, Matthew S. Kelly, Shane Crotty
Abstract
Maternal low thyroxine (T4) serum levels during the first trimester of pregnancy correlate with cerebral cortex volume and mental development of the progeny, but why neural cells during early fetal brain development are vulnerable to maternal T4 levels remains unknown. In this study, using iPSCs obtained from a boy with a loss-of-function mutation in MCT8—a transporter previously identified as critical for thyroid hormone uptake and action in neural cells—we demonstrate that thyroid hormones induce transcriptional changes that promote the progression of human neural precursor cells along the dorsal projection trajectory. Consistent with these findings, single-cell, spatial, and bulk transcriptomics from MCT8-deficient cerebral organoids and cultures of human neural precursor cells underscore the necessity for optimal thyroid hormone levels for these cells to differentiate into neurons. The controlled intracellular activation of T4 signaling occurs through the transient expression of the enzyme type 2 deiodinase, which converts T4 into its active form, T3, alongside the coordinated expression of thyroid hormone nuclear receptors. The intracellular activation of T4 in NPCs results in transcriptional changes important for their division mode and cell cycle progression. Thus, T4 is essential for fetal neurogenesis, highlighting the importance of adequate treatment for mothers with hypothyroidism.
Authors
Federico Salas-Lucia, Sergio Escamilla, Amanda Charest, Hanzi Jiang, Randy Stout, Antonio C. Bianco
Abstract
In vitro studies have implicated orphan receptor GPRC5B in β-cell survival, proliferation and insulin secretion, but its relevance for glucose homeostasis in vivo is largely unknown. Using tamoxifen-inducible, β-cell-specific GPRC5B knockout mice (Ins-G5b-KOs) we show here that loss of GPRC5B does not affect β-cell function in the lean state, but results in strongly reduced insulin secretion and disturbed glucose tolerance in mice subjected to high fat diet for 16 weeks. Flow cytometry and single-cell expression analyses in islets from obese mice show a reduced β-cell abundance and a less mature β-cell phenotype in Ins-G5b-KOs. Expression of β-cell-specific transcription factor MafA is reduced both on the RNA and protein level, as are transcripts of MafA target genes. Mechanistically, we show that phosphorylation of cAMP response element-binding protein (CREB), a major regulator of MafA expression, is reduced in islets of obese Ins-G5b-KOs, and that this phenotype precedes the downregulation of MafA and MafA target genes. Taken together, GPRC5B helps to maintain mature β-cell function in obesity through cAMP/CREB-dependent regulation of MafA expression.
Authors
Tianpeng Wang, Remy Bonnavion, Janett Piesker, Stefan Günther, Nina Wettschureck