Therapeutics
Abstract
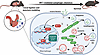
We recently showed that cell surface translocation of the endoplasmic reticulum–resident protein GRP78, when bound by activated α 2-macroglobulin (α2M*), induces pro-fibrotic responses in glomerular mesangial cells in response to high glucose and regulates activation of the pro-fibrotic cytokine transforming growth factor-β1 (TGF-β1), implicating a pathogenic role in glomerulosclerosis. Interstitial fibrosis, largely mediated by proximal tubular epithelial cells (PTEC) and renal fibroblasts, develops later in kidney disease and correlates with functional decline. Here we investigated whether interstitial fibrosis was mediated by cell surface GRP78 (csGRP78)/α2M*. High glucose and TGF-β1 increased csGRP78 and α2M* in PTEC and renal fibroblasts, and their inhibition prevented fibrotic protein production. Interestingly, for TGF-β1, this depended on inhibition of noncanonical signaling through YAP/TAZ, with Smad3 activation unaffected. In vivo, type 1 diabetic Akita mice overexpressing TGF-β1 were treated with either a neutralizing antibody for csGRP78 (C38) or α2M* (Fα2M) or an inhibitory peptide blocking csGRP78/α2M* interaction, and mice with unilateral ureteral obstruction were treated with Fα2M or inhibitory peptide. Consistently, inhibition by antibody or peptide attenuated fibrosis and pro-fibrotic signaling. These findings show an important role for csGRP78/α2M* in mediating tubulointerstitial fibrosis in both diabetic and nondiabetic kidney disease and support their inhibition as a potential antifibrotic therapeutic intervention.
Authors
Jackie Trink, Ifeanyi Kennedy Nmecha, Katrine Pilely, Renzhong Li, Zi Yang, Sydney Kwiecien, Melissa MacDonald, Bo Gao, Mariam A. Mamai, Chao Lu, Urooj F. Bajwa, Nikhil Uppal, James C. Fredenburgh, Masao Kakoki, Salvatore V. Pizzo, Anthony F. Rullo, Matthew B. Lanktree, Jeffrey I. Weitz, Yaseelan Palarasah, Joan C. Krepinsky
Abstract
Epigenetic macromolecular enzyme complexes tightly regulate gene expression at the chromatin level and have recently been found to colocalize with RNA splicing machinery during active transcription; however, the precise functional consequences of these interactions are uncertain. Here, we identify unique interactions of the CoREST repressor complex (LSD1-HDAC1-CoREST) with components of the RNA splicing machinery and their functional consequences in tumorigenesis. Using mass spectrometry, in vivo binding assays, and cryo-EM we find that CoREST complex-splicing factor interactions are direct and perturbed by the CoREST complex selective inhibitor, corin, leading to extensive changes in RNA splicing in melanoma and other malignancies. Moreover, these corin-induced splicing changes are shown to promote global effects on oncogenic and survival-associated splice variants leading to a tumor-suppressive phenotype. Using machine learning models, MHC IP-MS, and ELISpot assays we identify thousands of neopeptides derived from unannotated splice sites which generate corin-induced splice-neoantigens that are demonstrated to be immunogenic in vitro. Corin is further shown to reactivate the response to immune checkpoint blockade, effectively sensitizing tumors to anti-PD1 immunotherapy. These data position CoREST complex inhibition as a unique therapeutic opportunity which perturbs oncogenic splicing programs while also creating tumor-associated neoantigens that enhance the immunogenicity of current therapeutics.
Authors
Robert J. Fisher, Kihyun Park, Kwangwoon Lee, Katarina Pinjusic, Allison Vanasse, Christina S. Ennis, Parisa Farokh, Scott B. Ficarro, Jarrod A. Marto, Hanjie Jiang, Eunju Nam, Stephanie Stransky, Joseph Duke-Cohan, Melis A. Akinci, Anupa Geethadevi, Eric Raabe, Ana Fiszbein, Shadmehr Demehri, Simone Sidoli, Chad W. Hicks, Derin B. Keskin, Catherine J. Wu, Philip A. Cole, Rhoda M. Alani
Abstract
Sepsis contributes substantially to mortality rates worldwide, yet clinical trials that have focused on its underlying pathogenesis have failed to demonstrate benefits. Recently, enhancing self-defense has been regarded as an emerging therapeutic approach. Autophagy is a self-defense mechanism that protects septic mice, but its regulatory factor is still unknown. Moreover, the role of interferon regulatory factor 7 (IRF7) in sepsis has been debated. Here, we showed that Irf7 deficiency increased mortality during polymicrobial sepsis. Furthermore, IRF7 drove macrophages to protect against sepsis. Mechanistically, IRF7 is a transcription factor that upregulates the expression of autophagy-related genes responsible for autophagosome formation and autolysosome maturation, induces autophagic killing of bacteria, and ultimately reduces septic organ injury. Recombinant adeno-associated virus 9–Irf7–mediated IRF7 overexpression promoted the autophagic clearance of pathogens and improved sepsis outcomes, which may be the mechanism underlying the observed improvement in bacterial clearance. These findings provide evidence that IRF7 is the underlying regulatory factor that drives autophagy to eliminate pathogens in macrophages during sepsis. Collectively, IRF7 overexpression represents a potential host-directed therapeutic strategy for preclinical sepsis models, operating independently of antibiotic mechanisms.
Authors
Guiming Chen, Kangxin Li, Haihua Luo, Lianxu Zhao, Yong Jiang
Abstract
Anthracycline chemotherapy, widely used in cancer treatment, poses a significant risk of cardiotoxicity that results in functional decline. Current diagnostic methods poorly predict cardiotoxicity because they do not detect early damage that precedes dysfunction. Positron emission tomography (PET) is well-suited to address this need when coupled with suitable imaging biomarkers. We used PET to evaluate cardiac molecular changes in male C57BL/6J mice exposed to doxorubicin (DOX). These mice initially developed cardiac atrophy, experienced functional deficits within 10 weeks of treatment, and developed cardiac fibrosis by 16 weeks. Elevated cardiac uptake of [68Ga]Ga-FAPI-04, a PET tracer targeting fibroblast activation protein alpha (FAP), was evident by 2 weeks and preceded the onset of functional deficits. Cardiac PET signal correlated with FAP expression and activity as well as other canonical indicators of cardiac remodeling. By contrast, cardiac uptake of [18F]DPA-714 and [18F]MFBG, which target translocator protein 18-kDa (TSPO) and the norepinephrine transporter (NET), respectively, did not differ between the DOX animals and their controls. These findings identify FAP as an early imaging biomarker for DOX-induced cardiac remodeling in males and support the use of FAP PET imaging to detect some cancer patients at risk for treatment-related myocardial damage before cardiac function declines.
Authors
Chul-Hee Lee, Onorina L. Manzo, Luisa Rubinelli, Sebastian E. Carrasco, Sungyun Cho, Thomas M. Jeitner, John W. Babich, Annarita Di Lorenzo, James M. Kelly
Abstract
Butyrate, a microbiome-derived short-chain fatty acid with pleiotropic effects on inflammation and metabolism, has been shown to significantly reduce atherosclerotic lesions, rectify routine metabolic parameters such as low-density lipoprotein cholesterol (LDL-C), and reduce systemic inflammation in murine models of atherosclerosis. However, its foul odor, rapid metabolism in the gut and thus low systemic bioavailability limit its therapeutic effectiveness. Our laboratory has engineered an ester-linked L-serine conjugate to butyrate (SerBut) to mask its taste and odor and to coopt amino acid transporters in the gut to increase its systemic bioavailability, as determined by tissue measurements of free butyrate, produced by hydrolysis of SerBut. In an apolipoprotein E–knockout (ApoE)–/– mouse model of atherosclerosis, SerBut reduced systemic LDL-C, proinflammatory cytokines, and circulating neutrophils. SerBut enhanced inhibition of plaque progression and reduced monocyte accumulation in the aorta compared with sodium butyrate. SerBut suppressed liver injury biomarkers alanine transaminase and aspartate aminotransferase and suppressed steatosis in the liver. SerBut overcomes several barriers to the translation of butyrate and shows superior promise in slowing atherosclerosis and liver injury compared with equidosed sodium butyrate.
Authors
Taryn N. Beckman, Lisa R. Volpatti, Salvador Norton de Matos, Anna J. Slezak, Joseph W. Reda, Ada Weinstock, Leah Ziolkowski, Alex Turk, Erica Budina, Shijie Cao, Gustavo Borjas, Jung Woo Kwon, Orlando deLeon, Kirsten C. Refvik, Abigail L. Lauterbach, Suzana Gomes, Eugene B. Chang, Jeffrey A. Hubbell
Abstract
Glycogen storage disease type Ia (GSD Ia) is caused by a deficiency of glucose-6-phosphatase (G6Pase) in the liver leading to lethal hypoglycemia. Gene therapy with adeno-associated virus (AAV) vectors encoding G6Pase fails to stably treat GSD Ia early in life. We evaluated genome editing in 12 day-old infant mice with GSD Ia using two AAV vectors, one containing Cas9 from Streptococcus pyogenes and a second Donor vector that expresses a guide RNA and a G6PC transgene. Gene therapy with the Donor vector only was compared with genome editing using both Donor and CRISPR vectors. Treatment with genome editing (total vector dose 0.2 to 2E+13 vector genomes/kg) and bezafibrate (to stimulate autophagy) was efficacious as assessed by hypoglycemia prevention and the frequency of transgene integration, which correlated with improved survival. This therapy achieved 5.9% chromosomal transgene integration through homology directed repair, which surpassed a threshold to prevent long-term hepatic complications. No integration was detected in absence of the CRISPR vector. Importantly for safety, CRISPR vector genomes were depleted, and no intact, integrated CRISPR genomes were detected by long-read sequencing. Thus, genome editing warrants further development as a potentially stable treatment for human infants with GSD Ia.
Authors
Benjamin Arnson, Ekaterina Ilich, Troy von Beck, Songtao Li, Elizabeth D. Brooks, Dorothy Gheorghiu, Gordon He, Matthew Weinrub, Sze Ying Chan, Hye-Ri Kang, David Courtney, Jeffrey Everitt, Bryan R. Cullen, Dwight D. Koeberl
Abstract
Idiopathic pulmonary fibrosis (IPF) is a severe diffuse progressive fibrosing interstitial disease leading to respiratory failure and death in the absence of organ transplantation. Substantial evidence has confirmed the pivotal role of fibroblasts in the progression of IPF, yet effective therapeutic options are scarce. Single-cell transcriptomics profiling revealed that among the diverse fibroblast subsets, FAP1+ alveolar fibroblasts (AFs) are pivotal for the progression of IPF. On the basis of these findings, we developed FAP1-targeting chimeric antigen receptor cytotoxic effector regulatory T (CAR-cTregs) cells, which leverage the targeted killing advantage of the currently trending CAR-based immunotherapy for tumors and incorporate the immunosuppressive functions of Tregs to mitigate the inflammation caused by both the disease itself and CAR-T-cell infusion. Accordingly, CAR-cTregs were constructed to effectively eliminate FAP1+ fibroblasts in vitro. This cytotoxic effect can be abrogated by inhibitors of the granzyme-perforin pathway. In the bleomycin-induced PF model, CAR-cTregs were found to reverse fibrosis characterized by diminished recruitment of fibrocytes and improved remodeling of epithelial cells. Together, our results demonstrate that CAR-cTregs can serve as a promising therapeutic option for IPF and provide a novel strategy for treating multiple chronic inflammatory diseases by inducing both cytotoxicity and immunosuppression.
Authors
Yun-Han Jiang, Meng Zhou, Meng-Di Cheng, Sai Chen, Ying-Qiang Guo
Abstract
Androgen receptor positive prostate cancer (PC), castration resistant prostate cancer (CRPC) and neuroendocrine prostate cancer (NEPC) invariably become resistant to treatment with targeted and cytotoxic agents. Multiple pathways have been identified as being responsible for these pleotropic mechanisms of resistance. The MUC1 gene is aberrantly expressed in CRPC/NEPC in association with poor clinical outcomes; whereas, it is not known if the oncogenic MUC1-C/M1C protein drives treatment resistance. We demonstrated that MUC1-C is necessary for resistance of (i) PC cells to enzalutamide (ENZ), and (ii) CRPC and NEPC cells to docetaxel (DTX). Our results showed that MUC1-C-mediated resistance is conferred by upregulation of aerobic glycolysis and suppression of reactive oxygen species necessary for self-renewal. Dependence of these resistant phenotypes on MUC1-C for the cancer stem cell (CSC) state identified a potential target for treatment. In this regard, we further demonstrated that targeting MUC1-C with a M1C antibody-drug conjugate (ADC) is highly effective in suppressing (i) self-renewal of drug-resistant CRPC/NEPC CSCs and (ii) growth of t-NEPC tumor xenografts derived from drug-resistant cells and a patient with refractory disease. These findings uncovered a common MUC1-C-dependent pathway in treatment-resistant CRPC/NEPC progression and identified MUC1-C as a target for their treatment with a M1C ADC.
Authors
Keisuke Shigeta, Tatsuaki Daimon, Hiroshi Hongo, Sheng-Yu Ku, Hiroki Ozawa, Naoki Haratake, Atsushi Fushimi, Ayako Nakashoji, Atrayee Bhattacharya, Shinkichi Takamori, Michihisa Kono, Masahiro Rokugo, Yuto Baba, Takeo Kosaka, Mototsugu Oya, Justine Jacobi, Mark D. Long, Himisha Beltran, Donald W. Kufe
Abstract
Prion diseases are fatal, infectious and incurable neurodegenerative conditions affecting humans and animals, caused by the misfolding of the cellular prion protein (PrPC) into its pathogenic isoform, PrPSc. In humans, sporadic Creutzfeldt-Jakob disease (sCJD) is the most prevalent form. Recently, we demonstrated that treatment with the FDA-approved anti-HIV drug Efavirenz (EFV) significantly reduced PrPSc and extended survival of scrapie prion-infected mice. Among other effects, EFV activates the brain cholesterol metabolizing enzyme, CYP46A1, which converts cholesterol into 24S-hydroxycholesterol (24S-HC). However, drugs effective against scrapie prions often fail in human prion diseases, and a relation of the anti-prion effects of EFV to CYP46A1 activation is not established. Thus, we evaluated EFV treatment in mice overexpressing human PrPC infected with human sCJD prions. Oral, low-dose EFV treatment starting at 30- or 130-days post-infection significantly slowed disease progression and extended their survival. At early clinical stage, we observed reduced PrPSc accumulation, decreased cholesterol and lipid droplet content, and elevated CYP46A1 and 24S-HC levels in EFV-treated mice. Overexpression of CYP46A1 in prion-infected neuronal cells reduced PrPSc levels and increased 24S-HC, indicating that anti-prion effects of EFV correlate with CYP46A1 activation. These findings highlight EFV as a safe and efficacious therapeutic candidate for human prion diseases.
Authors
Tahir Ali, Jessica Cashion, Samia Hannaoui, Hanaa Ahmed-Hassan, Hermann M. Schatzl, Sabine Gilch
Abstract
Hypoxia-inducible factors (HIFs) promote lung protection and pathogen eradication during acute lung injury. We therefore tested the theory that pharmacologic stabilization of HIFs dampens lung injury during SARS-CoV-2 pneumonia. Initial studies in murine SARS-CoV-2 models showed improved outcomes after treatment with the FDA-approved HIF-stabilizer vadadustat. Subsequent studies in genetic models implicated alveolar-expressed Hif1a in mediating lung protection. Therefore, we performed a randomized, double-blinded, multicenter phase 2 trial in patients admitted for SARS-CoV-2 infection and concomitant hypoxia (SpO2 ≤ 94%). Patients (n=448) were randomized to oral vadadustat (900 mg/day) or placebo for up to 14 days. Safety events were similar between the two groups. Vadadustat treatment induced surrogate HIF-target genes. The primary outcome of severe lung injury requiring high oxygen support on day 14 occurred in 43 patients in the vadadustat group and 53 patients in the placebo group (estimated probability, 13.3% vs. 16.9%). Among patients with baseline FiO2 ≥ 80% (n=106), the estimated probability of the primary outcome was 12.1% (vadadustat) vs. 79.1% (placebo), indicating an even greater benefit in patients with more severe baseline hypoxia. HIF1A is a likely therapeutic target during SARS-CoV-2-associated lung injury. Robust clinical trials of HIF stabilizers during pathogen-associated lung injury are warranted.
Authors
Bentley Bobrow, Samuel D. Luber, Paul Potnuru, Katherine Figarella, Jieun Kim, Yanyu Wang, In Hyuk Bang, David Robinson, Paulina B. Sergot, Steven K. Burke, Tingting Mills, Constanza de Dios, Robert Suchting, George W. Williams, Adit A. Ginde, Yafen Liang, Hongfang Liu, Charles Green, Marie-Francoise Doursout, Alparslan Turan, Daniel I. Sessler, Xiaoyi Yuan, Holger K. Eltzschig
No posts were found with this tag.