Bone biology
Abstract
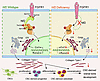
Adaptive remodeling of retrodiscal tissue following anterior disc displacement (ADD) of the temporomandibular joint (TMJ) has been recognized for decades, yet the underlying cellular dynamics and molecular mechanisms remain unclear. Using a porcine ADD model, this study investigated the cellular and molecular basis driving retrodiscal tissue adaptation. Histological staining revealed adaptive remodeling of retrodiscal tissue after ADD induction, with dense connective tissue and cartilaginous masses replacing loose connective tissue. Furthermore, single-cell RNA sequencing (scRNA-seq) captured pronounced fibroblast expansion during tissue remodeling, notably the FB2 subcluster with high developmental potential, and the emergence of a mural cell subcluster MC4 associated with extracellular matrix (ECM) remodeling. CellChat analysis highlighted MC4-FB2 crosstalk via FGF2 and BMP5 signaling. The combination of pathway-aware multi-layered hierarchical network (P-NET) and Seurat with drug database screening identified five promising compounds. Among them, Zaprinast demonstrated the most robust effects by enhancing the remodeling capability of fibroblasts in vitro, and also alleviated TMJ deformation in vivo. Collectively, fibroblast activation is pivotal for early retrodiscal tissue adaptation following ADD, which is driven by MC4-derived FGF2/BMP5 signaling. Zaprinast treatment potentiates this remodeling process. These findings provide new insights into cellular basis of TMJ adaptation and identify potential therapeutic targets for ADD management.
Authors
Wenlin Yuan, Yilin Chen, Ruojin Yan, Wei Liu, Chenyu Wang, Ying Wang, Qiaoli Dai, Wen Li, Mengqi Zhu, Xiao Chen, Jiejun Shi
Abstract
The TRPV4 skeletal dysplasias are characterized by short stature, short limbs with prominent large joints, and progressive scoliosis. They result from dominant missense mutations that activate the TRPV4 calcium permeable ion channel. As a platform to understand the mechanism of disease and to test the hypothesis that channel inhibition could treat these disorders, we developed a knock-in mouse that conditionally expresses the p.R594H Trpv4 mutation. Embryonic, chondrocyte-specific induction of the mutation using Col2a1-Cre resulted in a skeletal dysplasia affecting the long bones, spine, and craniofacial skeletal elements, consistent with the human skeletal dysplasia phenotypes produced by TRPV4 mutations. Cartilage growth plate histological abnormalities included disorganized proliferating chondrocyte columns and reduced hypertrophic chondrocyte development, reflecting abnormal endochondral ossification. In vivo treatment with the TRPV4-specific inhibitor GSK2798745 markedly improved the radiographic skeletal phenotype and rescued the growth plate histological abnormalities. ScRNA-Seq of chondrocyte transcripts from affected mice identified calcium-mediated effects on multiple signaling pathways as potential mechanisms underlying the defects in linear and cartilage appositional growth observed in both mutant mice and patients. These results provide preclinical evidence demonstrating TRPV4 inhibition as a rational, mechanism-based therapeutic strategy to ameliorate disease progression and severity in the TRPV4 skeletal dysplasias.
Authors
Lisette Nevarez, Taylor K. Ismaili, Jennifer Zieba, Jorge Martin, Davis Wachtell, Derick Diaz, Jocelyn A. Ramirez, Valeria Aceves, Joshua Ito, Ryan S. Gray, David Goldstein, Sunil Sahdeo, Deborah Krakow, Daniel H. Cohn
Abstract
Loss of bone mass has a devastating effect on quality of life. Higher potassium (K+) intake is positively correlated with bone health. Here, we investigated whether kidney calcium (Ca2+) and phosphate (Pi) handling mechanisms mediate dietary K+ effects. Kidney Ca2+ and Pi handling proteins were altered in abundance in mice fed a 0% K+ diet for 2 weeks. In mice fed a 0.1% K+ diet for 4 or 8 weeks, urinary Ca2+ excretion increased, plasma Ca2+ levels were lower and plasma parathyroid hormone (PTH) levels were higher relative to control 1% K+ fed mice. The 0.1% K+ fed mice had greater excretion of the bone resorption marker deoxypyridinoline, increased osteoclast number, and decreased total femoral bone mineral density. During chronic low K+ intake, major changes in renal Ca2+ and Pi transport pathways were absent, except higher abundances of the sodium-potassium-chloride co-transporter (NKCC2) and the sodium-chloride co-transporter (NCC), in line with their role in kidney Ca2+ handling. Low dietary K+ induced hypocalcemia and changes in PTH were absent in mice with constitutively active NCC, supporting its role in mediating low K+ effects on Ca2+ homeostasis. Our study provides insights into the management of bone disorders in conditions of chronic electrolyte imbalance.
Authors
Sathish K. Murali, Mariavittoria D'Acierno, Xiang Zheng, Lena K. Rosenbaek, Louise N. Odgaard, Paul Richard Grimm, Alice Ramesova, Robert Little, Judith Radloff, Paul A. Welling, Qi Wu, Reinhold G. Erben, Robert A. Fenton
Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive motor neuron disease. Emerging evidence suggests manifestations beyond the neuromuscular system. Bone alterations are part of the ALS clinical picture; it remains unclear whether they are secondary to muscle denervation or due to an autonomous process. We investigated skeletal involvement in the SOD1(G93A) mouse model at presymptomatic (P45) and symptomatic (P110) stage through biomechanical and transcriptomic approaches. Three-point bending revealed significant reductions in femoral rigidity and maximum bending force in SOD1 mutants at P45, indicating early structural deficits. Micro-CT analysis demonstrated reduced trabecular bone mineral density and thickness at P45, with progressive trabecular loss and cortical thinning by P110. Histological examination revealed marked osteoblast loss at P45 suggesting impaired bone formation as the primary early mechanism. Transcriptomics of bulk bone and cultured osteoblasts from P45 mice identified dysregulation of bone differentiation, including downregulation of osteoblast differentiation genes and upregulation of negative regulators of ossification and increased cell senescence signatures. Unfolded protein response was upregulated in SOD1 osteoblasts. Immunohistochemistry confirmed the senescence phenotype with increased p16Ink4a level in SOD1 osteoblasts. These findings suggest that bone deterioration precede overt motor symptoms and are linked to osteoblast premature senescence.
Authors
Burak Özkan, Jan-Moritz Ramge, Diana Wiesner, Jelena Scekic-Zahirovic, Stefano Antonucci, Sandra Nungeß, Dorothea Gebauer, Anita Ignatius, Jochen H. Weishaupt, Melanie Haffner-Luntzer, Francesco Roselli
Abstract
C-type natriuretic peptide (CNP) is known to promote chondrocyte proliferation and bone formation; however, CNP’s extremely short half-life necessitates continuous intravascular administration to achieve bone-lengthening effects. Vosoritide, a CNP analog designed for resistance to neutral endopeptidase, allows for once daily administration. Nonetheless, it distributes systemically rather than localizing to target tissues, which may result in adverse effects such as hypotension. To enhance local drug delivery and therapeutic efficacy, we developed a novel synthetic protein by fusing a collagen-binding domain (CBD) to CNP, termed CBD-CNP. This fusion protein exhibited stability under heat conditions and retained the collagen-binding ability and bioactivity as CNP. CBD-CNP localized to articular cartilage in fetal murine tibiae and promoted bone elongation. Spatial transcriptomic analysis revealed that the upregulation of chondromodulin expression may contribute to its therapeutic effects. Treatment of CBD-CNP mixed with collagen powder to a fracture site of a mouse model increased bone mineral content and bone volume rather than CNP-22. Intra-articular injection of CBD-CNP to a mouse model of knee osteoarthritis suppressed subchondral bone thickening. By addressing the limitations of CNP’s rapid degeneration, CBD-CNP leverages its collagen-binding capacity to achieve targeted, sustained delivery in collagen-rich tissues, offering a promising strategy for enhancing chondrogenesis and osteogenesis.
Authors
Kenta Hirai, Kenta Sawamura, Ryusaku Esaki, Ryusuke Sawada, Yuka Okusha, Eriko Aoyama, Hiroki Saito, Kentaro Uchida, Takehiko Mima, Satoshi Kubota, Hirokazu Tsukahara, Shiro Imagama, Masaki Matsushita, Osamu Matsushita, Yasuyuki Hosono
Abstract
Ehlers-Danlos Syndrome, Classic-Like, 2 (clEDS2) is a rare genetic disorder caused by biallelic mutations in the AEBP1 gene, which encodes Aortic carboxypeptidase-like protein (ACLP). Patients with clEDS2 exhibit hallmark features such as loose connective tissues, osteoporosis, and scoliosis. Despite its clinical significance, the molecular mechanisms underlying AEBP1 mutations in skeletal development remain poorly understood, and effective therapeutic strategies are currently unavailable. Here, using OsxCre conditional knockout mice, we show that Aebp1 deletion in osteoprogenitors reduces body size and bone mass, recapitulating key skeletal features reported in clEDS2. In primary osteoblasts, both genetic deletion and siRNA-mediated knockdown of Aebp1 impair osteoblast differentiation. Mechanistically, Aebp1 loss attenuates Wnt/β-catenin signaling in bone. Restoration of Wnt/β-catenin signaling by injecting BIO, a small molecule inhibitor of GSK3, substantially rescued bone mass reduction in Aebp1 knockout mice. These findings support a model in which Aebp1 sustains baseline Wnt/β-catenin tone in osteoblast-lineage cells and suggest that Wnt-targeted approaches may help mitigate clEDS2-related skeletal defects.
Authors
Shuhao Feng, Zihang Feng, Zhonghao Deng, Yiran Wei, Ru Lian, Yangchen Jin, Shiqi Zhao, Yu Jin, Zhongmin Zhang, Liang Zhao
Abstract
Modic type 1 and 2 changes (MC-1 and MC-2) are highly prevalent in individuals with chronic low back pain, yet the cellular and molecular mechanisms underlying vertebral endplate degeneration remain poorly defined. Here, we report that osteoclastogenesis is markedly elevated in MC-1 and MC-2 lesions compared to MC-3, suggesting an active role for osteoclasts in the early stages of degeneration. Using a lumbar spine instability (LSI) mouse model, we demonstrate enhanced osteoclast activity in degenerating endplates. RNA sequencing of mononuclear cells isolated from the endplate and adjacent subchondral bone identifies Gdf15 as a potential upstream regulator of this process. Conditional knockout of Gdf15 in monocytes reduces osteoclast formation, aberrant CD31hiEmcnhi angiogenesis, and pain-associated neurogenesis, ultimately mitigating endplate degeneration and mechanical allodynia. Mechanistically, GDF15 promotes the fusion of preosteoclasts by modulating the expression of Rho-family small GTPases. In a humanized GDF15 knock-in mouse model, therapeutic neutralization of GDF15 leads to a reduction in osteoclast burden, improved endplate structure, and attenuated pain behavior. Together, these findings uncover a previously unrecognized role for GDF15 in driving osteoclast-mediated endplate degeneration and highlight its potential as a therapeutic target for the treatment of endplate-related chronic low back pain.
Authors
Xiaoqun Li, Jinhui Wu, Qingjie Kong, Miao Hu, Yuhong Li, Ziheng Wei, Heng Jiang, Xuhui Zhou, Jun Ma
Abstract
Cranial neural crest cells (CNCs) play a critical role in craniofacial bone morphogenesis, engaging in intricate interactions with various molecular signals to ensure proper development, yet the molecular scaffolds coordinating these processes remain incompletely defined. Here, we identify neurofibromin 2 (Nf2) as a critical regulator to direct CNC-derived skull morphogenesis. Genetic ablation of Nf2 in murine CNCs causes severe craniofacial anomalies, featuring declined proliferation and increased apoptosis in osteoprogenitors, impaired type I collagen biosynthesis and trafficking, and aberrant osteogenic mineralization. Mechanistically, we uncover that Nf2 serves as a molecular linker that individually interacts with FGF receptor 1 (FGFR1) and Akt through spatially segregated phosphor-sites, and structural modeling and mutagenesis identified Ser10 and Thr230 as essential residues, with Thr230 mutation selectively ablating Akt binding while preserving FGFR1 association. Strikingly, Akt inhibition phenocopied Nf2 deficiency, reducing collagen production and Nf2 phosphorylation, whereas phospho-mimetic Nf2 (T230D) rescued CNC-derived osteogenic defects in Nf2-mutant animals. Our findings underscore the physiological significance of Nf2 as a phosphorylation-operated scaffold licensing the FGFR1/AKT axis to regulate collagen type I biogenesis and trafficking, ensuring normal CNC-derived osteogenesis and craniofacial bone development, thus exposing the Nf2/FGFR1/AKT signaling axis as a therapeutic target and promising advancements in treatment of craniofacial anomalies.
Authors
Yuping Huang, Junguang Liao, Panpan Shen, Yiliang He, Fuju Sun, Qi Zhang, Changlin Zheng, Xingen Zhang, Haibo Li, Guiqian Chen
Abstract
Talc pleurodesis is highly effective for preventing recurrence of pneumothorax and pleural effusion, but can be complicated by dissemination, acute lung injury, lead exposure, and foreign body-induced chronic inflammation and pain. Our objective is to develop a safe, biodegradable, contaminant-free particle for pleurodesis. We used mouse models of pneumothorax and malignant pleural effusion to compare the efficacy and safety of pleurodesis with talc and hydroxyapatite microspheres (HAM). Intrapleural instillation of microspheres induced pleural adhesions, fibrosis and symphysis as effectively as talc, and resulted in more durable protection from experimental pneumothorax. HAM and talc both induced an osteoclastogenic, inflammatory and fibrotic response in pleural lavage cells. Intrapleural HAM was resorbed by osteoclast action over 3 months, whereas talc was not cleared. Deletion of the osteoclast effector, CTSK, diminished pleural adhesion formation and fibrosis by talc and HAM, and inhibition of osteoclastogenesis with anti-RANKL antibody delayed HAM clearance. We found no difference in activity level, feeding behavior or lung compliance between particles, but talc induced more persistent pleural inflammation. We conclude that HAM resulted in an osteoclastogenic and fibrogenic pleural response that induced pleurodesis that was more durable than talc with a superior safety profile due in part to osteoclast-mediated particle clearance.
Authors
Yusuke Tanaka, Yuki Takahashi, Yuma Shindo, Lori B. Pitstick, Steven L. Teitelbaum, Wei Zou, Xiangning Wang, Jason Woods, Kathryn A. Wikenheiser-Brokamp, Francis X. McCormack
Abstract
Autophagy is a recycling pathway in which damaged proteins, protein aggregates, and organelles are delivered to lysosomes for degradation. Autophagy insufficiency is thought to contribute to osteoporosis. Accordingly, autophagy elimination from the osteoblast lineage reduces bone formation and bone mass. However, whether increasing autophagy would benefit bone health is unknown. Here, we increased expression of endogenous transcription factor EB gene (Tfeb) in osteoblast lineage cells in vivo via CRISPR activation (TfebCRa mice). Elevated Tfeb stimulated autophagy and lysosomal biogenesis in osteoblasts. TfebCRa mice displayed a robust increase in femoral and vertebral cortical thickness at 4.5 months of age. Increases in cortical thickness was due to increased periosteal bone formation. Tfeb elevation also increased femoral trabecular bone volume. These changes increased bone strength of TfebCRa mice. Female TfebCRa mice displayed a progressive increase in bone mass and at 12 months of age had high cortical thickness and trabecular bone volume. Increased vertebral trabecular bone volume was due to elevated bone formation. Osteoblastic cultures showed that Tfeb elevation increased proliferation and mineral deposition. Overall, these results demonstrate TFEB-driven stimulation of autophagy in osteoblast lineage cells is associated with increased bone formation and strength and may represent an effective approach to combat osteoporosis.
Authors
Alicen James, James A. Hendrixson, Ilham Kadhim, Adriana Marques-Carvalho, Jacob Laster, Julie Crawford, Jeff Thostenson, Visanu Wanchai, Amy Y. Sato, Intawat Nookaew, Jinhu Xiong, Maria Almeida, Melda Onal
No posts were found with this tag.