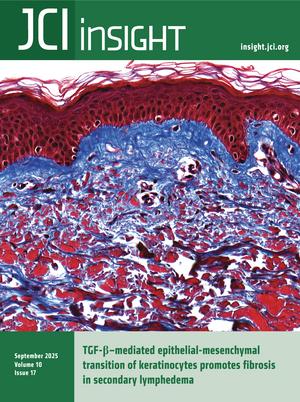
TGF-β–mediated epithelial-mesenchymal transition of keratinocytes promotes fibrosis in secondary lymphedema
Park et al. show that keratinocytes undergo epithelial-mesenchymal transition (EMT), which contributes to skin fibrosis in lymphedema in a TGF-β–dependent fashion. This cover image illustrates subepidermal fibrosis in lymphedema patient skin visualized by Mason’s trichrome staining. Image credit: Raghu P. Kataru and Hyeung Ju Park
-
Perspective
×
Abstract
The American Physician Scientists Association (APSA) was founded in 2003 with a mission to build a unified community for physician-scientist trainees. Over the past 2 decades, the APSA has played a pivotal role in fostering the development of future physician-scientists through mentorship, advocacy, and professional development. This year, the APSA hosted its 20th Annual Meeting in Chicago in collaboration with the Association of American Physicians and the American Society for Clinical Investigation. This milestone marks a moment of celebration and reflection, highlighting APSA’s enduring impact on the future of physician-scientist training.
Authors
Cynthia Y. Tang, Alex D. Waldman, Daniel C. Brock
-
Research Articles
×
Abstract
Bronchopulmonary dysplasia (BPD), a prevalent and chronic lung disease affecting premature newborns, results in vascular rarefaction and alveolar simplification. Although the vasculature has been recognized as a main player in this disease, the recently found capillary heterogeneity and cellular dynamics of endothelial subpopulations in BPD remain unclear. Here, we showed that Cap2 cells were damaged during neonatal hyperoxic injury, leading to their replacement by Cap1 cells, which, in turn, significantly declined. Single-cell RNA-Seq identified the activation of numerous p53 target genes in endothelial cells (ECs), including Cdkn1a (p21). While global deletion of p53 resulted in worsened vasculature, EC-specific deletion of p53 reversed the vascular phenotype and improved alveolar simplification during hyperoxia. This recovery was associated with the emergence of a transitional EC state, enriched for oxidative stress response genes and growth factors. Notably, this transitional EC gene signature was conserved in an aberrant capillary population identified in human BPD with pulmonary hypertension, underscoring the biological and clinical relevance of our findings. These results reveal a key role for p53 in maintaining endothelial lineage fidelity during pulmonary capillary repair following hyperoxic injury and highlight the critical contribution of the endothelium to BPD pathogenesis.
Authors
Lisandra Vila Ellis, Jonathan D. Bywaters, Amanda Ceas, Yun Liu, Jennifer M.S. Sucre, Jichao Chen
Bo Zhang, Prajish Iyer, Meiling Jin, Elisa Ten Hacken, Zachary J. Cartun, Kevyn L. Hart, Mike Fernandez, Kristen Stevenson, Laura Rassenti, Emanuela M. Ghia, Thomas J. Kipps, Donna Neuberg, Ruben Carrasco, Wing C. Chan, Joo Y. Song, Yu Hu, Catherine J. Wu, Lili WangBo Zhang, Prajish Iyer, Meiling Jin, Elisa Ten Hacken, Zachary J. Cartun, Kevyn L. Hart, Mike Fernandez, Kristen Stevenson, Laura Rassenti, Emanuela M. Ghia, Thomas J. Kipps, Donna Neuberg, Ruben Carrasco, Wing C. Chan, Joo Y. Song, Yu Hu, Catherine J. Wu, Lili Wang×Abstract
RNA splicing factor SF3B1 is one of the most recurrently mutated genes in chronic lymphocytic leukemia (CLL) and frequently co-occurs with chromosome 13q deletion [del(13q)]. This combination is associated with poor prognosis in CLL, suggesting these lesions increase CLL aggressiveness. While del(13q) in murine B cells (minimal deleted region of 13q14 includes DLEU1, DLEU2, and miR15a-16-1; Mdr mice), but not expression of Sf3b1-K700E, drives the initiation of CLL, we hypothesize that SF3B1 mutation accelerates CLL progression. In this study, we crossed mice with a B cell–specific Sf3b1-K700E allele with Mdr mice to determine the impact of Sf3b1 mutation on CLL progression. We found that the co-occurrence of these 2 lesions in murine B cells caused acceleration of CLL. We showed that Sf3b1-K700E impacted alternative RNA splicing of nuclear factor of activated T cells C1 (Nfatc1) and activated mTOR signaling and the MYC pathway, contributing to CLL acceleration. Moreover, concurrent inhibition of RNA splicing and the mTOR pathway led to cell death in vitro and in vivo in murine CLL cells with SF3B1 mutation and del(13q). Our results thus suggest that SF3B1 mutation contributes to the aggressiveness of CLL by activating the mTOR pathway through alternative splicing of Nfatc1, providing a rationale for targeting mTOR and RNA splicing in the subset of CLL patients with both SF3B1 mutations and del(13q).
Authors
Bo Zhang, Prajish Iyer, Meiling Jin, Elisa Ten Hacken, Zachary J. Cartun, Kevyn L. Hart, Mike Fernandez, Kristen Stevenson, Laura Rassenti, Emanuela M. Ghia, Thomas J. Kipps, Donna Neuberg, Ruben Carrasco, Wing C. Chan, Joo Y. Song, Yu Hu, Catherine J. Wu, Lili Wang
Wen-Chin Tsai, Chiu-Fen Yang, Shu-Yu Lin, Suh-Yuen Liang, Wei-Chung Tsai, Shuai Guo, Xiaochun Li, Susan Ofner, Kai-Chien Yang, Tzu-Ching Meng, Peng-Sheng Chen, Michael RubartWen-Chin Tsai, Chiu-Fen Yang, Shu-Yu Lin, Suh-Yuen Liang, Wei-Chung Tsai, Shuai Guo, Xiaochun Li, Susan Ofner, Kai-Chien Yang, Tzu-Ching Meng, Peng-Sheng Chen, Michael Rubart×Abstract
Heterozygosity for missense mutations in 1 of 3 seemingly redundant calmodulin-encoding (CALM-encoding) genes can cause life-threatening arrhythmias, suggesting that small fractions of mutant CALM protein suffice to cause a severe phenotype. However, the exact molar ratios of wild-type to mutant CALM protein in calmodulinopathy hearts remain unknown. The aim of the present study was to quantitate mutant versus wild-type CALM transcript and protein levels in hearts of knockin mice harboring the p.N98S mutation in the Calm1 gene. We found that the transcripts from the mutant Calm1 allele were the least abundantly expressed Calm transcripts in both hetero- and homozygous mutant hearts, while mutant hearts accumulated high levels of N98S-CALM protein in a Calm1N98S allele dosage-dependent manner, exceeding those of wild-type CALM protein. We further show that the severity of the electrophysiological phenotype incrementally increased with the graded increase in the mutant/wild-type CALM protein expression ratio seen in homozygous versus heterozygous mutant mice. We finally show a decrease in N98S-CALM protein degradation, suggesting that mutant CALM stabilization contributed to its enrichment in the heart. Our results support what we believe to be a novel mechanism by which a mutation in a single Calm gene can give rise to a severe phenotype.
Authors
Wen-Chin Tsai, Chiu-Fen Yang, Shu-Yu Lin, Suh-Yuen Liang, Wei-Chung Tsai, Shuai Guo, Xiaochun Li, Susan Ofner, Kai-Chien Yang, Tzu-Ching Meng, Peng-Sheng Chen, Michael Rubart
×Abstract
More than a third of patients with glioblastoma experience tumor progression during adjuvant therapy. In this study, we performed a high-throughput drug repurposing screen of FDA-approved agents capable of crossing the blood-brain barrier in order to find agents to counteract acquired or inherent glioma cell resistance to temozolomide-associated cytotoxicity. We identified the cholesterol processing inhibitor, lomitapide, as a potential chemosensitizer in glioblastoma. In vitro treatment of temozolomide-resistant glioblastoma cells with lomitapide resulted in decreased intracellular ubiquinone levels and sensitized cells to temozolomide-induced ferroptosis. Concomitant treatment with lomitapide and temozolomide (TMZ) prolonged survival and delayed tumor recurrence in a mouse glioblastoma model, compared with treatment xwith TMZ alone. Our data identified lomitapide as a potential adjunct for treatment of temozolomide-resistant glioblastoma.
Authors
Alyona Ivanova, Taylor M. Wilson, Kimia Ghannad-Zadeh, Esmond Tse, Robert Flick, Megan Wu, Sunit Das
Zhimin Huang, Jiaxin Dong, Ziqi Fu, Li Li, Simeng Liu, Lin Wu, Honglei Guo, Ao Bian, Kang Liu, Wei Sun, Changying Xing, Steven D Crowley, Jiafa Ren, Xiangqing Kong, Huijuan MaoZhimin Huang, Jiaxin Dong, Ziqi Fu, Li Li, Simeng Liu, Lin Wu, Honglei Guo, Ao Bian, Kang Liu, Wei Sun, Changying Xing, Steven D Crowley, Jiafa Ren, Xiangqing Kong, Huijuan Mao×Low-intensity pulsed ultrasound stimulation to treat renal fibrosis through inhibiting tubular IL-1R
Abstract
Low-intensity pulsed ultrasound stimulation (LIPUS) has become increasingly appreciated for its therapeutic effect on kidney diseases. However, its role and biological mechanism in treating chronic kidney disease remain poorly defined. Here, we revealed that LIPUS was applied in a safe range with an intensity of 25–315 mW/cm2. Daily LIPUS at an intensity of 315 mW/cm2 ameliorated ischemia/reperfusion-induced (IR-induced) tubular injury and renal fibrosis, accompanied by the remarkable downregulation of IL-1R. Transcriptome sequencing showed that LIPUS significantly downregulated IL-1R and its downstream genes in IL-1β–stimulated IR-injured mice. LIPUS effectively reversed IL-1β–induced tubular injury and reduced the production of pro-fibrotic cytokines by downregulating IL-1R in vivo and in vitro. Renal proximal tubule–specific Il1r1-knockout mice exhibited milder renal tubular injury and fibrosis after IR injury. However, LIPUS did not ameliorate IR injury in proximal tubule–specific Il1r1-knockout mice. Collectively, daily LIPUS at an intensity of 315 mW/cm2 relieves IR-induced tubular injury and fibrosis, potentially by downregulating tubular IL-1R.
Authors
Zhimin Huang, Jiaxin Dong, Ziqi Fu, Li Li, Simeng Liu, Lin Wu, Honglei Guo, Ao Bian, Kang Liu, Wei Sun, Changying Xing, Steven D Crowley, Jiafa Ren, Xiangqing Kong, Huijuan Mao
Qingxiao Song, Moqian Zheng, Qinjian Li, Xiwei Wu, Boxi Lin, Tae Hyuk Kang, Hanjun Qin, Maciej Kujawski, Raju K. Pillai, James L. Lin, Ryotaro Nakamura, John Shively, Paul J. Martin, Defu ZengQingxiao Song, Moqian Zheng, Qinjian Li, Xiwei Wu, Boxi Lin, Tae Hyuk Kang, Hanjun Qin, Maciej Kujawski, Raju K. Pillai, James L. Lin, Ryotaro Nakamura, John Shively, Paul J. Martin, Defu Zeng×Abstract
Steroid-refractory gut acute graft-versus-host disease (SR-Gut-aGVHD) is the major cause of nonrelapse death after allogeneic hematopoietic cell transplantation. High numbers of donor-type IL-22+ T cells, IL-22–dependent dysbiosis, and loss of antiinflammatory CX3CR1hi mononuclear phagocytes (MNPs) play critical roles in SR-Gut-aGVHD pathogenesis. CEACAM1 on intestinal epithelial cells (IECs) is proposed to regulate bacterial translocation and subsequent immune responses in the intestine. Here, with imaging mass cytometry (IMC), combined scRNA-Seq with ATAC-Seq, and high-dimensional flow cytometry analysis, we show that CEACAM1 expression was enhanced on IECs in murine and human SR-Gut-aGVHD. Ceacam1 deficiency on host IECs effectively prevented SR-Gut-aGVHD in murine models. Ceacam1 deficiency on IECs resulted in (i) higher numbers of IL-22+IL-10+Foxp3+CD4+ peripheral Tregs (pTregs) and lower numbers of conventional IL-22+CD4+ T (Tcon), Th/Tc1, and Th17 cells in the intestine; (ii) higher prevalence of beneficial commensal bacteria that augment colonic pTreg expansion, with lower prevalence of pathogenic bacteria; and (iii) higher numbers of antiinflammatory CD103–CX3CR1hi MNPs that produce indoleamine 2,3-dioxygenase (IDO) and IL-10, with lower numbers of proinflammatory CD103+CX3CR1lo MNPs that produce IL-6. Thus, specifically targeting IEC CEACAM1 represents a promising approach for prevention of SR-Gut-aGVHD.
Authors
Qingxiao Song, Moqian Zheng, Qinjian Li, Xiwei Wu, Boxi Lin, Tae Hyuk Kang, Hanjun Qin, Maciej Kujawski, Raju K. Pillai, James L. Lin, Ryotaro Nakamura, John Shively, Paul J. Martin, Defu Zeng
Yukun Yuan, Heather A. O’Malley, Jesse J. Winters, Alfonso Lavado, Nicholas S. Denomme, Shreeya Bakshi, Samantha L. Hodges, Luis Lopez-Santiago, Chunling Chen, Lori L. IsomYukun Yuan, Heather A. O’Malley, Jesse J. Winters, Alfonso Lavado, Nicholas S. Denomme, Shreeya Bakshi, Samantha L. Hodges, Luis Lopez-Santiago, Chunling Chen, Lori L. Isom×Abstract
Patients with Dravet syndrome (DS) present with severe, spontaneous seizures and ataxia. While most patients with DS have variants in the sodium channel Nav1.1 α subunit gene, SCN1A, variants in the sodium channel β1 subunit gene, SCN1B, are also linked to DS. Scn1b null mice model DS, with spontaneous generalized seizures that start in the second week of life. In Scn1b null cerebellum, neuronal pathfinding is severely altered, and Purkinje cells (PCs) and granule neurons have altered excitability. Here, we show that Scn1b null mice are ataxic. Expression of β1 protein in WT cerebellum, assessed using a CRISPR transgenic mouse model containing an in-frame V5 epitope tag at the β1 C-terminus, is widespread. Scn1b null PCs and interneurons in cerebellar slices have increased thresholds for action potential initiation and decreased repetitive firing frequency compared with WT. Scn1b null PCs have reduced transient and resurgent sodium current densities. We propose that reduced PC excitability underlies the ataxic phenotype of Scn1b mice. In addition, because cerebellar output to other areas of the brain can result in termination of seizures, we propose that PC hypoexcitability exacerbates the severe phenotype of this mouse model.
Authors
Yukun Yuan, Heather A. O’Malley, Jesse J. Winters, Alfonso Lavado, Nicholas S. Denomme, Shreeya Bakshi, Samantha L. Hodges, Luis Lopez-Santiago, Chunling Chen, Lori L. Isom
Madeline G. Hemmingsen, Guo-Fang Zhang, Yunhan Ma, Hannah Marchuk, Kalyani R. Patel, Tong Chen, Xinning Li, Mark Chapman, Sabrina Collias, Dolores H. Lopez-Terrada, James Beasley, Ashlee R. Stiles, Randy J. Chandler, Charles P. Venditti, Sarah P. Young, Mercedes Barzi, Beatrice Bissig-Choisat, Doug Krafte, Christopher B. Newgard, Karl-Dimiter BissigMadeline G. Hemmingsen, Guo-Fang Zhang, Yunhan Ma, Hannah Marchuk, Kalyani R. Patel, Tong Chen, Xinning Li, Mark Chapman, Sabrina Collias, Dolores H. Lopez-Terrada, James Beasley, Ashlee R. Stiles, Randy J. Chandler, Charles P. Venditti, Sarah P. Young, Mercedes Barzi, Beatrice Bissig-Choisat, Doug Krafte, Christopher B. Newgard, Karl-Dimiter Bissig×Abstract
Methylmalonic acidemia (MMA) is a severe metabolic disorder affecting multiple organs because of a distal block in branched-chain amino acid (BCAA) catabolism. Standard of care is limited to protein restriction and supportive care during metabolic decompensation. Severe cases require liver/kidney transplantation, and there is a clear need for better therapy. Here, we investigated the effects of a small molecule branched-chain amino acid transaminase (BCAT) inhibitor in human MMA hepatocytes and an MMA mouse model. Mitochondrial BCAT is the first step in BCAA catabolism, and reduction of flux through an early enzymatic step is successfully used in other amino acid metabolic disorders. Metabolic flux analyses confirmed robust BCAT inhibition, with reduction of labeling of proximal and distal BCAA-derived metabolites in MMA hepatocytes. In vivo experiments verified the BCAT inhibition, but total levels of distal BCAA catabolite disease markers and clinical symptoms were not normalized, indicating contributions of substrates other than BCAA to these distal metabolite pools. Our study demonstrates the importance of understanding the underlying pathology of metabolic disorders for identification of therapeutic targets and the use of multiple, complementary models to evaluate them.
Authors
Madeline G. Hemmingsen, Guo-Fang Zhang, Yunhan Ma, Hannah Marchuk, Kalyani R. Patel, Tong Chen, Xinning Li, Mark Chapman, Sabrina Collias, Dolores H. Lopez-Terrada, James Beasley, Ashlee R. Stiles, Randy J. Chandler, Charles P. Venditti, Sarah P. Young, Mercedes Barzi, Beatrice Bissig-Choisat, Doug Krafte, Christopher B. Newgard, Karl-Dimiter Bissig
Benjamin Cai, Rabina Giri, Amy J. Cameron, M. Arifur Rahman, Annabelle Small, Christopher Altmann, Yenkai Lim, Linda M. Rehaume, Mark Morrison, Mihir D. Wechalekar, Jakob Begun, Anne-Sophie Bergot, Ranjeny ThomasBenjamin Cai, Rabina Giri, Amy J. Cameron, M. Arifur Rahman, Annabelle Small, Christopher Altmann, Yenkai Lim, Linda M. Rehaume, Mark Morrison, Mihir D. Wechalekar, Jakob Begun, Anne-Sophie Bergot, Ranjeny Thomas×Abstract
Spondyloarthritis (SpA) is an inflammatory arthritis of the spine and joints associated with intestinal inflammation, in which it is hypothesized that innate immune exposure to enteroinvasive species is followed by self-/bacterial peptide presentation. However, the mechanisms underlying loss of tolerance to gut bacteria in genetically at-risk individuals are unclear. Curdlan-treated (β-1,3-glucan, dectin-1 ligand–treated) ZAP-70W163C (SKG) mice develop autoimmune arthritis and ileitis associated with Gram-negative fecal dysbiosis. Using gnotobiotic mice, we show that curdlan-treated SKG mice monoassociated with Parabacteroides goldsteinii or Lactobacillus murinus developed ileitis, arthritis, and enthesitis, while BALB/c mice were tolerant. Gnotobiotic SKG ileum upregulated Il23a and ER stress genes and lost goblet cells. Whereas bacterial DNA colocalized with neutrophils and inflammatory macrophages in SKG lamina propria, periarticular bone marrow, entheses, and spleen, in BALB/c mice, bacterial DNA colocalized with resident macrophages in lamina propria and spleen. Human psoriatic-arthritis synovial tissue also contained cell-associated perivascular bacterial DNA. Curdlan-treated SKG spleen/bone marrow macrophages transferred severe arthritis and expanded Th17 cells in naive SKG recipients, while BALB/c or germ-free SKG macrophages transferred mild arthritis and regulated Th17 cells. Thus, bacterial DNA and myeloid cells in the gut and their subsequent traffic regulate or enforce T cell pathogenicity in SpA.
Authors
Benjamin Cai, Rabina Giri, Amy J. Cameron, M. Arifur Rahman, Annabelle Small, Christopher Altmann, Yenkai Lim, Linda M. Rehaume, Mark Morrison, Mihir D. Wechalekar, Jakob Begun, Anne-Sophie Bergot, Ranjeny Thomas
Elisabet Bjånes, Nishta Krishnan, Truman Koh, Anh T.P. Ngo, Jason Cole, Joshua Olson, Ingrid Cornax, Chih-Ho Chen, Natalie Chavarria, Samira Dahesh, Shawn M. Hannah, Alexandra Stream, Jiaqi Amber Zhang, Hervé Besançon, Daniel Sun, Siri Yendluri, Sydney Morrill, Jiarong Zhou, Animesh Mohapatra, Ronnie H. Fang, Victor NizetElisabet Bjånes, Nishta Krishnan, Truman Koh, Anh T.P. Ngo, Jason Cole, Joshua Olson, Ingrid Cornax, Chih-Ho Chen, Natalie Chavarria, Samira Dahesh, Shawn M. Hannah, Alexandra Stream, Jiaqi Amber Zhang, Hervé Besançon, Daniel Sun, Siri Yendluri, Sydney Morrill, Jiarong Zhou, Animesh Mohapatra, Ronnie H. Fang, Victor Nizet×Abstract
Multidrug-resistant (MDR) bacterial pneumonia poses a critical threat to global public health. The opportunistic Gram-negative pathogen Pseudomonas aeruginosa is a leading cause of nosocomial-associated pneumonia, and an effective vaccine could protect vulnerable populations, including the elderly, immunocompromised, and those with chronic respiratory diseases. Highly heterogeneous outer membrane vesicles (OMVs), shed from Gram-negative bacteria, are studded with immunogenic lipids, proteins, and virulence factors. To overcome limitations in OMV stability and consistency, we described what we believe to be a novel vaccine platform that combines immunogenic OMVs with precision nanotechnology — creating a bacterial cellular nanoparticle (CNP) vaccine candidate, termed Pa-STING CNP, which incorporates an adjuvanted core that activates the STING (stimulator of interferon genes) pathway. In this design, OMVs are coated onto the surface of self-adjuvanted STING nanocores. Pa-STING CNP vaccination induced substantial antigen presenting cell recruitment and activation in draining lymph nodes, robust anti-Pseudomonas antibody responses, and provided protection against lethal challenge with the hypervirulent clinical P. aeruginosa isolate PA14. Antibody responses mediated this protection and provided passive immunity against the heterologous P. aeruginosa strain PA01. These findings provided evidence that nanotechnology can be used to create a highly efficacious vaccine platform against high priority MDR pathogens such as P. aeruginosa.
Authors
Elisabet Bjånes, Nishta Krishnan, Truman Koh, Anh T.P. Ngo, Jason Cole, Joshua Olson, Ingrid Cornax, Chih-Ho Chen, Natalie Chavarria, Samira Dahesh, Shawn M. Hannah, Alexandra Stream, Jiaqi Amber Zhang, Hervé Besançon, Daniel Sun, Siri Yendluri, Sydney Morrill, Jiarong Zhou, Animesh Mohapatra, Ronnie H. Fang, Victor Nizet
Timothy J. Cashman, Sherin Saheera, Ashley E. Blau, Edith Mensah Otabil, Nouran Y. Nagy, Thomas D. Samenuk, Timothy P. Fitzgibbons, David D. McManus, Chinmay M. TrivediTimothy J. Cashman, Sherin Saheera, Ashley E. Blau, Edith Mensah Otabil, Nouran Y. Nagy, Thomas D. Samenuk, Timothy P. Fitzgibbons, David D. McManus, Chinmay M. Trivedi×Abstract
Aortic valve stenosis is a progressive and increasingly prevalent disease in older adults, with no approved pharmacologic therapies to prevent or slow its progression. Although genetic risk factors have been identified, the contribution of epigenetic regulation remains poorly understood. Here, we demonstrated that histone deacetylase 3 (HDAC3) maintains aortic valve structure by suppressing mitochondrial biogenesis and preserving extracellular matrix integrity in valvular interstitial fibroblasts. Human stenotic valves displayed elevated acetylation of histone H3 at lysine 27 (H3K27ac) and reduced HDAC3 activity in diseased regions. Mice lacking HDAC3 in aortic valves developed aortic valve stenosis, disrupted collagen organization, increased H3K27ac, and premature mortality. Mechanistically, HDAC3 loss led to activation of nuclear hormone receptor–regulated mitochondrial gene programs, increased oxidative phosphorylation, and reactive oxygen species–induced damage. Treatment with metformin, a mitochondrial complex I inhibitor, restored redox balance, preserved collagen structure, and improved valve function in Hdac3-deficient mice. Supporting these experimental findings, retrospective clinical analysis revealed a significantly lower prevalence and slower progression of aortic valve stenosis in patients treated with metformin. These results uncovered a potentially previously unrecognized role for HDAC3 in coordinating epigenetic and metabolic homeostasis in the aortic valve, suggesting that targeting mitochondrial dysfunction may offer a therapeutic strategy for noncalcific aortic valve disease.
Authors
Timothy J. Cashman, Sherin Saheera, Ashley E. Blau, Edith Mensah Otabil, Nouran Y. Nagy, Thomas D. Samenuk, Timothy P. Fitzgibbons, David D. McManus, Chinmay M. Trivedi
Petra Wilgenbus, Jennifer Pott, Sven Pagel, Claudius Witzler, Jennifer Royce, Federico Marini, Sabine Reyda, Thati Madhusudhan, Thomas Kindler, Anne Hausen, Matthias M. Gaida, Hartmut Weiler, Wolfram Ruf, Claudine GrafPetra Wilgenbus, Jennifer Pott, Sven Pagel, Claudius Witzler, Jennifer Royce, Federico Marini, Sabine Reyda, Thati Madhusudhan, Thomas Kindler, Anne Hausen, Matthias M. Gaida, Hartmut Weiler, Wolfram Ruf, Claudine Graf×Abstract
Malignancies increase the risk for thrombosis and metastasis dependent on complex interactions of innate immune cells, platelets, and the coagulation system. Immunosuppressive functions of platelets and macrophage-derived coagulation factors in the tumor microenvironment (TME) drive tumor growth. Here, we show that patients with malignancies and tumor-bearing mice have increased levels of coagulation factor (F) X–expressing circulating monocytes engaged in platelet aggregate formation. This interaction and resulting thrombin generation on platelets interferes with monocyte differentiation and antigen uptake of antigen-presenting cells (APCs). Myeloid cell–specific deletion of FX or abrogated FXa signaling via protease activated receptor 2 (PAR2) averts the suppressive activity of platelets on tumor cell debris uptake and promotes the immune stimulatory activity of APCs in the TME. Myeloid cell FXa-PAR2 signaling deficiency specifically enhances activation of the cGAS-STING-IFN-I pathway with a resulting expansion of antigen experienced progenitor exhausted CD8+ T cells. Pharmacological blockade of FXa with direct oral anticoagulants expands T cell priming–competent immune cells in the TME and synergizes with the reactivation of exhausted CD8+ T cells by immune checkpoint inhibitors for improved antitumor responses. These data provide mechanistic insights into the emerging clinical evidence demonstrating the translational potential of FXa inhibition to synergize with immunotherapy.
Authors
Petra Wilgenbus, Jennifer Pott, Sven Pagel, Claudius Witzler, Jennifer Royce, Federico Marini, Sabine Reyda, Thati Madhusudhan, Thomas Kindler, Anne Hausen, Matthias M. Gaida, Hartmut Weiler, Wolfram Ruf, Claudine Graf
Siavash Mansouri, Annika Karger, Clemens Ruppert, Marc A. Schneider, Andreas Weigert, Rajender Nandigama, Blerina Aliraj, Lisa Strotmann, Anoop V. Cherian, Diethard Pruefer, Peter Dorfmuller, Ludger Fink, Ibrahim Alkoudmani, Stefan Gattenlöhner, Bastian Eul, Andre Althoff, Peter Kleine, Hauke Winter, Andreas Guenther, Hossein-Ardeschir Ghofrani, Soni S. Pullamsetti, Friedrich Grimminger, Werner Seeger, Rajkumar SavaiSiavash Mansouri, Annika Karger, Clemens Ruppert, Marc A. Schneider, Andreas Weigert, Rajender Nandigama, Blerina Aliraj, Lisa Strotmann, Anoop V. Cherian, Diethard Pruefer, Peter Dorfmuller, Ludger Fink, Ibrahim Alkoudmani, Stefan Gattenlöhner, Bastian Eul, Andre Althoff, Peter Kleine, Hauke Winter, Andreas Guenther, Hossein-Ardeschir Ghofrani, Soni S. Pullamsetti, Friedrich Grimminger, Werner Seeger, Rajkumar Savai×Abstract
The tumor microenvironment (TME) markedly affects cancer progression, yet traditional animal models do not fully recapitulate the situation in humans. To address this, we developed tumor-derived precision lung slices (TD-PCLS), an ex vivo platform for studying the lung TME and evaluating therapies. TD-PCLS, viable for 8–10 days, preserve the heterogeneity and metabolic activity of primary tumors, as confirmed by seahorse analysis. Using multispectral FACS and phenocycler multiplex imaging, we spatially profiled TME components and cancer cell functionality. Additionally, TD-PCLS revealed patient-specific responses to chemo- and immunotherapies. To complement TD-PCLS, we established tumor-cell–seeded PCLS (TCS-PCLS) by introducing tumor and immune cells into healthy lung slices. This model highlighted macrophage-tumor interactions as critical for tumor cell proliferation, migration, and immune modulation. Together, these platforms provide a robust tool for lung cancer research, enabling precision medicine and advancing therapeutic discovery.
Authors
Siavash Mansouri, Annika Karger, Clemens Ruppert, Marc A. Schneider, Andreas Weigert, Rajender Nandigama, Blerina Aliraj, Lisa Strotmann, Anoop V. Cherian, Diethard Pruefer, Peter Dorfmuller, Ludger Fink, Ibrahim Alkoudmani, Stefan Gattenlöhner, Bastian Eul, Andre Althoff, Peter Kleine, Hauke Winter, Andreas Guenther, Hossein-Ardeschir Ghofrani, Soni S. Pullamsetti, Friedrich Grimminger, Werner Seeger, Rajkumar Savai
Roberto Ramos-Mondragon, Shuyun Wang, Nnamdi Edokobi, Qinghua Liu, Xiaotan Qiao, Maya Shih, Louis T. Dang, Yao-Chang Tsan, Katalin Štěrbová, Adam S. Helms, Sarah Weckhuysen, Luis F. Lopez-Santiago, Jack M. Parent, Lori L. IsomRoberto Ramos-Mondragon, Shuyun Wang, Nnamdi Edokobi, Qinghua Liu, Xiaotan Qiao, Maya Shih, Louis T. Dang, Yao-Chang Tsan, Katalin Štěrbová, Adam S. Helms, Sarah Weckhuysen, Luis F. Lopez-Santiago, Jack M. Parent, Lori L. Isom×Abstract
Biallelic variants in SCN1B, which encodes the voltage-gated sodium channel β1/β1B subunits, are linked to DEE52, a developmental and epileptic encephalopathy with a high risk of sudden unexpected death in epilepsy (SUDEP). DEE52 patients present clinically with Dravet syndrome or the more severe early infantile DEE. SCN1B is expressed in brain and heart in humans and in mice. Thus, we have proposed that, in addition to generalized seizures, cardiac arrhythmia may play a role in SUDEP. Mice with homozygous expression of the DEE52 variant Scn1b-c.265C>T, predicting p.R89C, have spontaneous and hyperthermia-induced generalized seizures and SUDEP. Here we conducted cardiac characterization of Scn1b-c.265C>T mice and studied induced pluripotent stem cell cardiomyocytes (iPSC-CMs) derived from 2 SCN1B-c.265C>T DEE52 patients. Scn1bC89/C89 mouse CMs showed increased transient outward potassium current (Ito) density and heart sections revealed ventricular fibrosis. Scn1bC89/C89 mice were susceptible to pacing-induced cardiac arrhythmias. Patient-derived iPSC-CMs with biallelic SCN1B-c.265C>T variant expression showed increased sodium current (INa), late INa, and Ito current densities. We conclude that, while mouse and human cardiac AP waveforms have critical differences, increased Ito is common to both models of DEE52. Overall, our data suggest that electrical and structural substrates may lead to arrhythmias and contribute to SUDEP in DEE52.
Authors
Roberto Ramos-Mondragon, Shuyun Wang, Nnamdi Edokobi, Qinghua Liu, Xiaotan Qiao, Maya Shih, Louis T. Dang, Yao-Chang Tsan, Katalin Štěrbová, Adam S. Helms, Sarah Weckhuysen, Luis F. Lopez-Santiago, Jack M. Parent, Lori L. Isom
Amalia Tzoumpa, Beatriz Lozano-Ruiz, Yin Huang, Joanna Picó, Alba Moratalla, María Teresa Pomares, Iván Herrera, Juanjo Lozano, María Rodríguez, Cayetano Miralles, Pablo Bellot, Paula Piñero, Fabián Tarín, Pedro Zapater, Sonia Pascual, José Manuel González-NavajasAmalia Tzoumpa, Beatriz Lozano-Ruiz, Yin Huang, Joanna Picó, Alba Moratalla, María Teresa Pomares, Iván Herrera, Juanjo Lozano, María Rodríguez, Cayetano Miralles, Pablo Bellot, Paula Piñero, Fabián Tarín, Pedro Zapater, Sonia Pascual, José Manuel González-Navajas×Abstract
BACKGROUND Liver cirrhosis is characterized by chronic inflammation and fibrosis, with Th17 cells playing a crucial role in its progression. Recent evidence suggests that dietary salt influences immune diseases by modulating Th17 differentiation. This study assessed the impact of dietary salt on Th17-driven inflammation in patients with compensated cirrhosis and explored its effects on liver injury in mouse models.METHODS A nondrug, open-label, nonrandomized study involved 37 patients with compensated cirrhosis, who were given personalized guidelines to reduce salt intake over 3 months. Changes in Th17-driven inflammation and liver function markers were assessed at baseline and after salt restriction. In parallel, the impact of a high-salt diet on hepatic CD4+ T cells was analyzed in mouse models of acute liver injury and fibrosis.RESULTS High salt intake was associated with Th17-mediated inflammation and correlated with markers of impaired liver function in these patients. Importantly, moderating salt intake through a personalized nutritional intervention was sufficient to reduce CD4+ T cell–mediated inflammation. Furthermore, analysis of RNA-seq data revealed enrichment of salt-induced Th17 gene signatures in both liver tissue and peripheral cells from patients with liver disease. Similarly, mice fed a high salt diet showed hepatic enrichment of Th17 cells and exacerbated liver fibrosis upon injury. Mechanistic studies revealed that high sodium conditions activated NF-κB and induced IL-6 production in hepatocytes, which may promote Th17 responses.CONCLUSION Dietary salt exacerbates Th17-driven inflammation and contributes to cirrhosis progression. Salt reduction may represent a viable therapeutic approach to manage inflammation in compensated cirrhosis.FUNDING Grants PI19/01554 and PI22/01907 from Instituto de Salud Carlos III (Madrid, Spain), CDEI-03/20-A and CIPROM/2023/4 from Generalitat Valenciana (Valencia, Spain), CNS2023-145676 from the National Research Agency (AEI) (Madrid, Spain), and LCF/BQ/D121/11860047 from La Caixa Foundation (Barcelona, Spain).
Authors
Amalia Tzoumpa, Beatriz Lozano-Ruiz, Yin Huang, Joanna Picó, Alba Moratalla, María Teresa Pomares, Iván Herrera, Juanjo Lozano, María Rodríguez, Cayetano Miralles, Pablo Bellot, Paula Piñero, Fabián Tarín, Pedro Zapater, Sonia Pascual, José Manuel González-Navajas
×Abstract
Transforming growth factor β (TGF-β) signaling is the master modulator of renal fibrosis. However, targeting drugs have failed to prevent the progression of chronic kidney disease (CKD) in clinical trials due to the extensive biological regulation of TGF-β signaling. It is necessary to investigate the precise downstream mechanisms of TGF-β signaling that regulate renal fibrosis. In this study, we found that PR-domain containing 16 (PRDM16) expression in human renal tubular epithelial cells was markedly reduced by TGF-β. Mechanistically, activated Smad3 induced by TGF-β interacted with the cofactor H-Ras and bound to the promoter of PRDM16, downregulating its transcription. Tubular-specific knockout of Prdm16 promoted renal fibrosis in models of unilateral ureteral occlusion (UUO) and unilateral ischemia-reperfusion injury (UIRI) by exacerbating mitochondrial dysfunction. In vitro, PRDM16 blocked TGF-β–induced mitochondrial injury and lipid deposition by upregulating peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α). Delivery of the exogenous PRDM16 gene preserved renal function and ameliorated the progression of renal fibrosis by protecting mitochondrial function. We report PRDM16 as a potential downstream target of TGF-β signaling that attenuates renal fibrosis by safeguarding tubular mitochondrial function.
Authors
Qian Yuan, Ben Tang, Yuting Zhu, Chao Wan, Yaru Xie, Yajuan Xie, Cheng Wan, Hua Su, Youhua Liu, Chun Zhang
Alicen James, James A. Hendrixson, Ilham Kadhim, Adriana Marques-Carvalho, Jacob Laster, Julie Crawford, Jeff Thostenson, Visanu Wanchai, Amy Y. Sato, Intawat Nookaew, Jinhu Xiong, Maria Almeida, Melda OnalAlicen James, James A. Hendrixson, Ilham Kadhim, Adriana Marques-Carvalho, Jacob Laster, Julie Crawford, Jeff Thostenson, Visanu Wanchai, Amy Y. Sato, Intawat Nookaew, Jinhu Xiong, Maria Almeida, Melda Onal×Abstract
Autophagy is a recycling pathway in which damaged proteins, protein aggregates, and organelles are delivered to lysosomes for degradation. Autophagy insufficiency is thought to contribute to osteoporosis. Accordingly, autophagy elimination from the osteoblast lineage reduces bone formation and bone mass. However, whether increasing autophagy would benefit bone health is unknown. Here, we increased expression of endogenous transcription factor EB gene (Tfeb) in osteoblast lineage cells in vivo via CRISPR activation (TfebCRa mice). Elevated Tfeb stimulated autophagy and lysosomal biogenesis in osteoblasts. TfebCRa mice displayed a robust increase in femoral and vertebral cortical thickness at 4.5 months of age. Increases in cortical thickness were due to increased periosteal bone formation. Tfeb elevation also increased femoral trabecular bone volume. These changes increased bone strength of TfebCRa mice. Female TfebCRa mice displayed a progressive increase in bone mass and at 12 months of age had high cortical thickness and trabecular bone volume. Increased vertebral trabecular bone volume was due to elevated bone formation. Osteoblastic cultures showed that Tfeb elevation increased proliferation and mineral deposition. Overall, these results demonstrate TFEB-driven stimulation of autophagy in osteoblast lineage cells is associated with increased bone formation and strength and may represent an effective approach to combat osteoporosis.
Authors
Alicen James, James A. Hendrixson, Ilham Kadhim, Adriana Marques-Carvalho, Jacob Laster, Julie Crawford, Jeff Thostenson, Visanu Wanchai, Amy Y. Sato, Intawat Nookaew, Jinhu Xiong, Maria Almeida, Melda Onal
×Abstract
Intracellular trafficking of secretory and membrane proteins from the endoplasmic reticulum (ER) to the cell surface, via the secretory pathway, is crucial to the differentiated function of epithelial tissues. In the thyroid gland, a prerequisite for such trafficking is proper protein folding in the ER, assisted by an array of ER molecular chaperones. One of the most abundant of these chaperones, Glucose-Regulated-Protein-170 (GRP170, encoded by Hyou1), is a noncanonical hsp70-like family member. Thyroid follicular epithelial cells abundantly express GRP170, but the role of this abundant ER chaperone in thyrocytes remains unknown. Here, we have examined the effect of inducible Pax8-specific (thyroid and kidney) deficiency of GRP170 in mice, in parallel with siRNA-treated PCCL3 (rat) thyrocytes for knockdown of GRP170. Thyrocyte-specific loss of GRP170 in vivo triggers primary hypothyroidism with a deficient thyroidal response to Thyroid-Stimulating Hormone (TSH). In addition, knockdown of GRP170 in PCCL3 thyrocytes inhibits the folding and forward trafficking of TSH receptors to the cell surface. Taken together, our findings suggest that GRP170 contributes to the conformational maturation of TSH receptors and thyroid gland responsiveness to TSH, which is required for proper regulation of thyroid hormone synthesis.
Authors
Xiaohan Zhang, Crystal Young, Xiao-Hui Liao, Samuel Refetoff, Stephanie M. Mutchler, Jeffrey L. Brodsky, Teresa M. Buck, Peter Arvan
×Abstract
Psoriasis is a chronic autoimmune skin disease characterized by abnormal keratinocyte proliferation and immune dysregulation. Altered lipid metabolism has been implicated in its pathogenesis, but the underlying mechanisms remain unclear. In this study, we generated a keratinocyte-specific Sprouty RTK signaling antagonist 1 (SPRY1) knockout (Spry1ΔEpi) mouse model, which exhibits psoriasis-like symptoms. Using both psoriasis patient samples and Spry1ΔEpi mice, we investigated the role of diacylglycerol acyltransferase 2 (DGAT2) in psoriasis. Our results show that DGAT2 expression was reduced and glyceride metabolism was disrupted in psoriatic lesions in both patients with psoriasis and Spry1ΔEpi mice. Lipidomic analysis revealed significant alterations in glycerides, glycerophospholipids, sphingolipids, and fatty acids in Spry1ΔEpi mice. At the cellular level, DGAT2 downregulation and lipid dysregulation enhanced TLR3-mediated inflammatory signaling in keratinocytes. Furthermore, increased DGAT2 secretion from keratinocytes promoted CD8+ T cell activation, proliferation, and survival, amplifying psoriatic inflammation. These findings highlight the role of DGAT2 and lipid metabolism in the pathogenesis of psoriasis and reveal their interaction with immune responses in psoriasis.
Authors
Ying-Ying Li, Li-Ran Ye, Ying-Zhe Cui, Fan Xu, Xi-Bei Chen, Feng-Fei Zhang, Yi Lu, Yu-Xin Zheng, Xiao-Yong Man
Hyeung Ju Park, Jinyeon Shin, Ananta Sarker, Mark G. Klang, Elyn Riedel, Michelle Coriddi, Joseph H. Dayan, Sarit Pal, Babak J. Mehrara, Raghu P. KataruHyeung Ju Park, Jinyeon Shin, Ananta Sarker, Mark G. Klang, Elyn Riedel, Michelle Coriddi, Joseph H. Dayan, Sarit Pal, Babak J. Mehrara, Raghu P. Kataru×Abstract
Secondary lymphedema is characterized by fibrosis and impaired lymphatic function. Although TGF-β is a key regulator of fibrosis in this disease, the cellular mechanisms regulating this process remain unknown. Epithelial-mesenchymal transition (EMT), a mechanism by which TGF-β induces fibrosis in other skin diseases, is characterized by loss of epithelial cell markers and cellular polarity, upregulation of fibrotic gene expression, and gain of migratory capacity. Using clinical lymphedema biopsy specimens and animal models, we show that keratinocytes in the basal layer of the epidermis undergo EMT in lymphedematous skin, migrate into the dermis, and contribute to dermal fibrosis. In vitro studies using cultured primary human keratinocytes treated with lymphatic fluid from the affected limbs of patients with secondary lymphedema resulted in a TGF-β–mediated increased expression of EMT markers. We show for the first time that EMT is activated by TGF-β in secondary lymphedema and that this process plays an important role in regulating skin fibrosis in this disease.
Authors
Hyeung Ju Park, Jinyeon Shin, Ananta Sarker, Mark G. Klang, Elyn Riedel, Michelle Coriddi, Joseph H. Dayan, Sarit Pal, Babak J. Mehrara, Raghu P. Kataru
Emma M. Pearce, Erica Evans, Madeline Y. Mayday, Gustavo Reyes, Miriam R. Simon, Jacob Blum, Hanna Kim, Jessica Mu, Peter J. Shaw, Courtney M. Rowan, Jeffery J. Auletta, Paul L. Martin, Caitlin Hurley, Erin M. Kreml, Muna Qayed, Hisham Abdel-Azim, Amy K. Keating, Geoffrey D.E. Cuvelier, Janet R. Hume, James S. Killinger, Kamar Godder, Rabi Hanna, Christine N. Duncan, Troy C. Quigg, Paul Castillo, Nahal R. Lalefar, Julie C. Fitzgerald, Kris M. Mahadeo, Prakash Satwani, Theodore B. Moore, Benjamin Hanisch, Aly Abdel-Mageed, Dereck B. Davis, Michelle P. Hudspeth, Greg A. Yanik, Michael A. Pulsipher, Christopher C. Dvorak, Joseph L. DeRisi, Matt S. ZinterEmma M. Pearce, Erica Evans, Madeline Y. Mayday, Gustavo Reyes, Miriam R. Simon, Jacob Blum, Hanna Kim, Jessica Mu, Peter J. Shaw, Courtney M. Rowan, Jeffery J. Auletta, Paul L. Martin, Caitlin Hurley, Erin M. Kreml, Muna Qayed, Hisham Abdel-Azim, Amy K. Keating, Geoffrey D.E. Cuvelier, Janet R. Hume, James S. Killinger, Kamar Godder, Rabi Hanna, Christine N. Duncan, Troy C. Quigg, Paul Castillo, Nahal R. Lalefar, Julie C. Fitzgerald, Kris M. Mahadeo, Prakash Satwani, Theodore B. Moore, Benjamin Hanisch, Aly Abdel-Mageed, Dereck B. Davis, Michelle P. Hudspeth, Greg A. Yanik, Michael A. Pulsipher, Christopher C. Dvorak, Joseph L. DeRisi, Matt S. Zinter×Abstract
Hematopoietic stem cell transplantation (HCT) is a potentially life-saving therapy but can lead to lung injury due to chemoradiation toxicity, infection, and immune dysregulation We previously showed that bronchoalveolar lavage (BAL) transcriptomes representing pulmonary inflammation and cellular injury can phenotype post-HCT lung injury and predict mortality. To test whether peripheral blood might be a suitable surrogate for BAL, we compared 210 paired BAL and blood transcriptomes obtained from 166 pediatric patients with HCT at 27 hospitals. BAL and blood RNA abundance showed minimal correlation at the level of individual genes, gene set enrichment scores, imputed cell fractions, and T and B cell receptor clonotypes. Instead, we identified significant site-specific transcriptional programs. In BAL, pathways related to immunity, hypoxia, and epithelial mesenchymal transition were tightly coexpressed and linked to mortality. In contrast, in blood, expression of endothelial injury, DNA repair, and cellular metabolism pathways was associated with mortality. Integration of paired BAL and blood transcriptomes dichotomized patients into 2 groups with significantly different rates of hypoxia and clinical outcomes within 1 week of BAL. These findings reveal a compartmentalized injury response, where BAL and blood transcriptomes provide distinct but complementary insights into local and systemic mechanisms of post-HCT lung injury.
Authors
Emma M. Pearce, Erica Evans, Madeline Y. Mayday, Gustavo Reyes, Miriam R. Simon, Jacob Blum, Hanna Kim, Jessica Mu, Peter J. Shaw, Courtney M. Rowan, Jeffery J. Auletta, Paul L. Martin, Caitlin Hurley, Erin M. Kreml, Muna Qayed, Hisham Abdel-Azim, Amy K. Keating, Geoffrey D.E. Cuvelier, Janet R. Hume, James S. Killinger, Kamar Godder, Rabi Hanna, Christine N. Duncan, Troy C. Quigg, Paul Castillo, Nahal R. Lalefar, Julie C. Fitzgerald, Kris M. Mahadeo, Prakash Satwani, Theodore B. Moore, Benjamin Hanisch, Aly Abdel-Mageed, Dereck B. Davis, Michelle P. Hudspeth, Greg A. Yanik, Michael A. Pulsipher, Christopher C. Dvorak, Joseph L. DeRisi, Matt S. Zinter

























