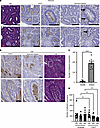
Soluble CD13 is a potential mediator of neutrophil-induced thrombogenic inflammation in SARS-CoV-2 infection
Tsou et al. report that the inflammatory mediator soluble CD13 (sCD13) is markedly elevated in serum and lungs of patients with SARS-CoV-2 and is a trigger of immunothrombosis in these patients. The cover image shows CD13 staining (green) in lungs from COVID-19 patients.
-
Research Letter
×
Abstract
Authors
Junping Yin, Melanie Eichler, Clivia Lisowski, Jian Li, Sibylle von Vietinghoff, Natalio Garbi, Qi Mei, Anne-Kathrin Gellner, Christian Kurts
-
Research Articles
Nina Bögershausen, Büsranur Cavdarli, Taylor H. Nagai, Miroslav P. Milev, Alexander Wolff, Mahsa Mehranfar, Julia Schmidt, Dharmendra Choudhary, Óscar Gutiérrez-Gutiérrez, Lukas Cyganek, Djenann Saint-Dic, Arne Zibat, Karl Köhrer, Tassilo E. Wollenweber, Dagmar Wieczorek, Janine Altmüller, Tatiana Borodina, Dilek Kaçar, Göknur Haliloğlu, Yun Li, Christian Thiel, Michael Sacher, Ela W. Knapik, Gökhan Yigit, Bernd WollnikNina Bögershausen, Büsranur Cavdarli, Taylor H. Nagai, Miroslav P. Milev, Alexander Wolff, Mahsa Mehranfar, Julia Schmidt, Dharmendra Choudhary, Óscar Gutiérrez-Gutiérrez, Lukas Cyganek, Djenann Saint-Dic, Arne Zibat, Karl Köhrer, Tassilo E. Wollenweber, Dagmar Wieczorek, Janine Altmüller, Tatiana Borodina, Dilek Kaçar, Göknur Haliloğlu, Yun Li, Christian Thiel, Michael Sacher, Ela W. Knapik, Gökhan Yigit, Bernd Wollnik×
Abstract
As a major component of intracellular trafficking, the coat protein complex II (COPII) is indispensable for cellular function during embryonic development and throughout life. The 4 SEC24 proteins (A–D) are essential COPII components involved in cargo selection and packaging. A human disorder corresponding to alterations of SEC24 function is currently known only for SEC24D. Here, we reported that biallelic loss of SEC24C leads to a syndrome characterized by primary microcephaly, brain anomalies, epilepsy, hearing loss, liver dysfunction, anemia, and cataracts in an extended consanguineous family with 4 affected individuals. We showed that knockout of sec24C in zebrafish recapitulated important aspects of the human phenotype. SEC24C-deficient fibroblasts displayed alterations in the expression of several COPII components as well as impaired anterograde trafficking to the Golgi, indicating a severe impact on COPII function. Transcriptome analysis revealed that SEC24C deficiency also affected the proteasome and autophagy pathways. Moreover, a shift in the N-glycosylation pattern and deregulation of the N-glycosylation pathway suggested a possible secondary alteration of protein glycosylation, linking the described disorder with the congenital disorders of glycosylation.
Authors
Nina Bögershausen, Büsranur Cavdarli, Taylor H. Nagai, Miroslav P. Milev, Alexander Wolff, Mahsa Mehranfar, Julia Schmidt, Dharmendra Choudhary, Óscar Gutiérrez-Gutiérrez, Lukas Cyganek, Djenann Saint-Dic, Arne Zibat, Karl Köhrer, Tassilo E. Wollenweber, Dagmar Wieczorek, Janine Altmüller, Tatiana Borodina, Dilek Kaçar, Göknur Haliloğlu, Yun Li, Christian Thiel, Michael Sacher, Ela W. Knapik, Gökhan Yigit, Bernd Wollnik
Kate Williamson, Katie J. Lee, Emma L. Beamish, Alan Carter, Jade A. Gumbs, Gabriella Cooper, Niamh S. O’Heneghan-Yates, Lisa A. Menezes, Graham Cheung, Daniel Brown, Rob Pettitt, Brendan Geraghty, Lucy A. Bosworth, Eithne J. Comerford, Peter D. Clegg, Elizabeth G. Canty-LairdKate Williamson, Katie J. Lee, Emma L. Beamish, Alan Carter, Jade A. Gumbs, Gabriella Cooper, Niamh S. O’Heneghan-Yates, Lisa A. Menezes, Graham Cheung, Daniel Brown, Rob Pettitt, Brendan Geraghty, Lucy A. Bosworth, Eithne J. Comerford, Peter D. Clegg, Elizabeth G. Canty-Laird×Abstract
Dupuytren’s disease is a common fibroproliferative disease of the palmar fascia of the hand, with advanced cases treated surgically. Anti-TNF injection has undergone phase 2 trials and may be effective in slowing early-stage disease progression. Here we sought to determine how new synthesis of type I collagen in Dupuytren’s differs from normal palmar fascia samples and to analyze the role of TNF in aberrant collagen synthesis. Model nonfibrotic but fibrous connective tissues were used to analyze active type I collagen protein synthesis in development, aging, and degenerative disease, where it was restricted to early development and ruptured tissue. Dupuytren’s tissue was shown to actively synthesize type I collagen, including abnormal type I collagen homotrimer. TNF-α reduced COL1A2 gene expression only in the presence of serum in 2D cell culture and had opposing effects on collagen protein production in the presence or absence of serum. TNF-α had only limited effects in 3D tendon–like constructs. Anti-TNF did not reduce type I collagen synthesis in 3D tendon–like constructs or prevent type I collagen homotrimer synthesis in Dupuytren’s tissue. Hence, modulation of the TNF-α pathway in Dupuytren’s disease is unlikely to prevent the pathological collagen accumulation that is characteristic of fibrosis.
Authors
Kate Williamson, Katie J. Lee, Emma L. Beamish, Alan Carter, Jade A. Gumbs, Gabriella Cooper, Niamh S. O’Heneghan-Yates, Lisa A. Menezes, Graham Cheung, Daniel Brown, Rob Pettitt, Brendan Geraghty, Lucy A. Bosworth, Eithne J. Comerford, Peter D. Clegg, Elizabeth G. Canty-Laird
×Abstract
Despite the accumulation of cisplatin in proximal tubules, direct visualization of the surrounding peritubular microcirculation, including its change in cisplatin-induced acute kidney injury (AKI), is lacking. Here, using fluorescence and cellular angiography through video-rate high-resolution intravital microscopy, progressive disturbance of peritubular microcirculation in cisplatin-induced AKI in mice was demonstrated. Fluorescence angiography revealed increasing perfusion defects, with a stepwise rise in time to peak (TTP), originating from capillaries surrounding S1 segments. Cellular angiography demonstrated a progressive decrease in the velocity and track length of individual erythrocytes during AKI progression, accompanied by a sequential decrease in the functional capillary ratio (FCR). Changes in the perfusion area, TTP, and FCR preceded significant changes in blood urea nitrogen and cystatin C, suggesting the potential for early diagnosis. Although neutrophil infiltration near proximal tubules increased throughout the progression, it did not cause obstruction of the peritubular microcirculation. Depletion of neutrophils increased mortality due to systemic side effects, whereas functional inactivation of neutrophils using an anti-CD11b antibody improved peritubular microcirculation in cisplatin-induced AKI. This approach enables direct visualization and quantification of peritubular microcirculation and immune cell dynamics, providing insights into renal pathophysiology and potential therapeutic strategies.
Authors
Inwon Park, Seonghye Kim, Young Woo Um, Hee Eun Kim, Jae Hyuk Lee, Sejoong Kim, Pilhan Kim, You Hwan Jo
Sarah A. McNitt, Jenna K. Dick, Maria Andrea Hernandez-Castaneda, Jules Sangala, Mark Pierson, Marissa Macchietto, Kristina S. Burrack, Peter D. Crompton, Karl Seydel, Sara E. Hamilton, Geoffrey T. HartSarah A. McNitt, Jenna K. Dick, Maria Andrea Hernandez-Castaneda, Jules Sangala, Mark Pierson, Marissa Macchietto, Kristina S. Burrack, Peter D. Crompton, Karl Seydel, Sara E. Hamilton, Geoffrey T. Hart×Abstract
P.falciparum infection can trigger high levels of inflammation that lead to fever and sometimes severe disease. People living in malaria-endemic areas gradually develop resistance to symptomatic malaria and control both parasite numbers and the inflammatory response. We previously found that adaptive NK cells correlated with reduced parasite load and protection from symptoms. We also found that murine NK cell production of IL-10 protected mice from experimental cerebral malaria. Human NK cells can also secrete IL-10, but it is unknown what NK cell subsets produce IL-10 or if this is affected by malaria experience. We hypothesized that NK cell immunoregulation may lower inflammation and reduce fever induction. Here, we showed that NK cells from participants with malaria experience make significantly more IL-10 than participants with no malaria experience. We then determined the proportions of NK cells that are cytotoxic and produce IFN-γ and/or IL-10 and identified a signature of adaptive and checkpoint molecules on IL-10–producing NK cells. Lastly, we found that coculture with primary monocytes, Plasmodium-infected RBCs, and antibody induced IL-10 production by NK cells. These data suggest that NK cells may contribute to protection from malaria symptoms via IL-10 production.
Authors
Sarah A. McNitt, Jenna K. Dick, Maria Andrea Hernandez-Castaneda, Jules Sangala, Mark Pierson, Marissa Macchietto, Kristina S. Burrack, Peter D. Crompton, Karl Seydel, Sara E. Hamilton, Geoffrey T. Hart
Paula M. Fraczek, Pamela Duran, Benjamin A. Yang, Valeria Ferre, Leanne Alawieh, Jesus A. Castor-Macias, Vivian T. Wong, Steve D. Guzman, Celeste Piotto, Klimentini Itsani, Jacqueline A. Larouche, Carlos A. AguilarPaula M. Fraczek, Pamela Duran, Benjamin A. Yang, Valeria Ferre, Leanne Alawieh, Jesus A. Castor-Macias, Vivian T. Wong, Steve D. Guzman, Celeste Piotto, Klimentini Itsani, Jacqueline A. Larouche, Carlos A. Aguilar×Abstract
Adult stem cells decline in number and function in old age, and identifying factors that can delay or revert age-associated adult stem cell dysfunction are vital for maintaining a healthy lifespan. Here we show that vitamin A, a micronutrient that is derived from diet and metabolized into retinoic acid, acts as an antioxidant and transcriptional regulator in muscle stem cells. We first show that obstruction of dietary vitamin A in young animals drives mitochondrial and cell cycle dysfunction in muscle stem cells that mimics old age. Next, we pharmacologically targeted retinoic acid signaling in myoblasts and aged muscle stem cells ex vivo and in vivo and observed reductions in oxidative damage, enhanced mitochondrial function, and improved maintenance of quiescence through fatty acid oxidation. We next detected that the receptor for vitamin A–derived retinol, stimulated by retinoic acid 6 or Stra6, was diminished with muscle stem cell activation and in old age. To understand the relevance of Stra6 loss, we knocked down Stra6 and observed an accumulation of mitochondrial reactive oxygen species, as well as changes in mitochondrial morphology and respiration. These results demonstrate that vitamin A regulates mitochondria and metabolism in muscle stem cells and highlight a unique mechanism connecting stem cell function with vitamin intake.
Authors
Paula M. Fraczek, Pamela Duran, Benjamin A. Yang, Valeria Ferre, Leanne Alawieh, Jesus A. Castor-Macias, Vivian T. Wong, Steve D. Guzman, Celeste Piotto, Klimentini Itsani, Jacqueline A. Larouche, Carlos A. Aguilar
Haremaru Kubo, Junta Imai, Tomohito Izumi, Masato Kohata, Yohei Kawana, Akira Endo, Hiroto Sugawara, Junro Seike, Takahiro Horiuchi, Hiroshi Komamura, Toshihiro Sato, Shinichiro Hosaka, Yoichiro Asai, Shinjiro Kodama, Kei Takahashi, Keizo Kaneko, Hideki KatagiriHaremaru Kubo, Junta Imai, Tomohito Izumi, Masato Kohata, Yohei Kawana, Akira Endo, Hiroto Sugawara, Junro Seike, Takahiro Horiuchi, Hiroshi Komamura, Toshihiro Sato, Shinichiro Hosaka, Yoichiro Asai, Shinjiro Kodama, Kei Takahashi, Keizo Kaneko, Hideki Katagiri×Abstract
Under insulin-resistant conditions, such as obesity, pancreatic β cells adaptively proliferate and secrete more insulin to prevent blood glucose elevation. We previously reported hepatic ERK activation during obesity development to stimulate a neuronal relay system, consisting of afferent splanchnic nerves from the liver and efferent vagal nerves to the pancreas, thereby triggering adaptive β cell proliferation. However, the mechanism linking obesity with the interorgan system originating in hepatic ERK activation remains unclear. Herein, we clarified that colonic inflammation promotes β cell proliferation through this interorgan system from the liver to the pancreas. First, dextran sodium sulfate (DSS) treatment induced colonic inflammation and hepatic ERK activation as well as β cell proliferation, all of which were suppressed by blockades of the neuronal relay system by several approaches. In addition, treatment with anti–lymphocyte Peyer’s patch adhesion molecule-1 (anti-LPAM1) antibody suppressed β cell proliferation induced by DSS treatment. Importantly, high-fat diet (HFD) feeding also elicited colonic inflammation, and its inhibition by anti-LPAM1 antibody administration suppressed hepatic ERK activation and β cell proliferation induced by HFD. Thus, colonic inflammation triggers adaptive β cell proliferation via the interorgan mechanism originating in hepatic ERK activation. The present study revealed a potentially novel role of the gastrointestinal tract in the maintenance of β cell regulation.
Authors
Haremaru Kubo, Junta Imai, Tomohito Izumi, Masato Kohata, Yohei Kawana, Akira Endo, Hiroto Sugawara, Junro Seike, Takahiro Horiuchi, Hiroshi Komamura, Toshihiro Sato, Shinichiro Hosaka, Yoichiro Asai, Shinjiro Kodama, Kei Takahashi, Keizo Kaneko, Hideki Katagiri
×Abstract
The soluble variant of the ectopeptidase CD13 (sCD13), released from the cell surface by matrix metalloproteinase 14 (MMP14), is a potent pro-inflammatory mediator, displaying chemotactic, angiogenic, and arthritogenic properties through bradykinin receptor B1 (B1R). We revealed a link between sCD13 and amplified neutrophil-mediated inflammatory responses in SARS-CoV-2 infection. sCD13 was markedly elevated in patients with COVID-19 and correlated with disease severity and variants, ethnicity, inflammation markers, and neutrophil extracellular trap formation (NETosis). Neutrophils treated with sCD13 showed heightened NETosis and chemotaxis, which were inhibited by sCD13 receptor blockade. Meanwhile sCD13 did not induce platelet aggregation. Single-cell analysis of COVID-19 lungs revealed coexpression of CD13 and MMP14 by various cell types, and higher CD13 expression compared with controls. Neutrophils with high CD13 mRNA were enriched for genes associated with immaturity, though CD13 protein expression was lower. Histological examination of COVID-19 lungs revealed CD13-positive leukocytes trapped in vessels with fibrin thrombi. Flow cytometry verified the presence of B1R and a second sCD13 receptor, protease-activated receptor 4, on monocytes and neutrophils. These findings identify sCD13 as a potential instigator of COVID-19–associated NETosis, potentiating vascular stress and thromboembolic complications. The potent pro-inflammatory effects of sCD13 may contribute to severe COVID-19, suggesting that sCD13 and its receptors might be therapeutic targets.
Authors
Pei-Suen Tsou, Ramadan A. Ali, Chenyang Lu, Gautam Sule, Carmelo Carmona-Rivera, Serena Lucotti, Yuzo Ikari, Qi Wu, Phillip L. Campbell, Mikel Gurrea-Rubio, Kohei Maeda, Sharon E. Fox, William D. Brodie, Megan N. Mattichak, Caroline Foster, Ajay Tambralli, Srilakshmi Yalavarthi, M. Asif Amin, Katarina Kmetova, Bruna Mazetto Fonseca, Emily Chong, Yu Zuo, Michael D. Maile, Luisa Imberti, Arnaldo Caruso, Francesca Caccuri, Virginia Quaresima, Alessandra Sottini, Douglas B. Kuhns, Danielle Fink, Riccardo Castagnoli, Ottavia M. Delmonte, Heather Kenney, Yu Zhang, Mary Magliocco, Helen Su, Luigi Notarangelo, Rachel L. Zemans, Yang Mao-Draayer, Irina R. Matei, Mirella Salvatore, David Lyden, Yogendra Kanthi, Mariana J. Kaplan, Jason S. Knight, David A. Fox
Sahar Mazhar, Caitlin M. O’Connor, Alexis Harold, Amanda C. Dowdican, Peter J. Ulintz, Erika N. Hanson, Yuping Zhang, Michelle F. Jacobs, Sofia D. Merajver, Mark W. Jackson, Anthony Scott, Anieta M. Sieuwerts, Arul M. Chinnaiyan, Goutham NarlaSahar Mazhar, Caitlin M. O’Connor, Alexis Harold, Amanda C. Dowdican, Peter J. Ulintz, Erika N. Hanson, Yuping Zhang, Michelle F. Jacobs, Sofia D. Merajver, Mark W. Jackson, Anthony Scott, Anieta M. Sieuwerts, Arul M. Chinnaiyan, Goutham Narla×Abstract
An estimated 5%–10% of cancer results from an underlying genetic predisposition. For the majority of familial cases, the genes in question remain unknown, suggesting a critical need to identify new cancer predisposition genes. Members of the protein phosphatase 2A (PP2A) family exist as trimeric holoenzymes and are vital negative regulators of multiple oncogenic pathways. PP2A inhibition by somatic mutation, loss of expression, and upregulation of its exogenous inhibitors in tumors has been well described. However, it remains unknown whether germline loss of any PP2A subunits results in a predisposition to cancer in humans. In this study, we identified 9 cancer patients with germline loss-of-function (LOF) variants in PPP2R1B (Aβ), the β isoform of the PP2A scaffold subunit. All 4 patients for whom documentation was available also had a family history of cancer, including multiple indicators of hereditary cancer. Overexpression of these mutant forms of Aβ resulted in truncated proteins that were rapidly turned over. Characterization of an additional missense germline Aβ variant, R233C, which is also recurrently mutated at the somatic level, showed disruption of PP2A catalytic subunit binding, resulting in loss of phosphatase activity. An analysis of Aβ expression among multiple breast cancer cohorts (the most highly represented cancer among the Aβ germline patients) revealed that somatic, heterozygous loss of Aβ was a frequent event in this disease, and decreased Aβ expression correlated with shorter disease-free and overall survival. Furthermore, Aβ levels were significantly lower in multiple histological subtypes of both in situ and malignant breast cancer compared with adjacent normal breast tissue, suggesting that Aβ loss is an early event in breast cancer development. Together, these results highlight a role for Aβ as a predisposition gene in breast cancer and potentially additional cancers.
Authors
Sahar Mazhar, Caitlin M. O’Connor, Alexis Harold, Amanda C. Dowdican, Peter J. Ulintz, Erika N. Hanson, Yuping Zhang, Michelle F. Jacobs, Sofia D. Merajver, Mark W. Jackson, Anthony Scott, Anieta M. Sieuwerts, Arul M. Chinnaiyan, Goutham Narla
Shanshan Jia, Weidong Xie, Chunqing Yang, Yizhang Dong, Wenting Luo, Hui Gu, Xiaowei Wei, Wei Ma, Dan Liu, Songying Cao, Yuzuo Bai, Wei Li, Zhengwei YuanShanshan Jia, Weidong Xie, Chunqing Yang, Yizhang Dong, Wenting Luo, Hui Gu, Xiaowei Wei, Wei Ma, Dan Liu, Songying Cao, Yuzuo Bai, Wei Li, Zhengwei Yuan×Abstract
Nonsyndromic cleft lip with palate (nsCLP) is a common birth defect disease. Current diagnostic methods comprise fetal ultrasound images, which are mainly limited by fetal position and technician skills. We aimed to identify reliable maternal serum lipid biomarkers to diagnose nsCLP. Eight-feature selection methods were used to assess the dysregulated lipids from untargeted lipidomics in a discovery cohort. The robust rank aggregation algorithm was applied on these selected lipids. The data were subsequently processed using 7 classification models to retrieve a panel of 35 candidate lipid biomarkers. Potential lipid biomarkers were evaluated using targeted lipidomics in a validation cohort. Seven classification models and multivariate analyses were constructed to identify the lipid biomarkers for nsCLP. The diagnostic model achieved high performance with 3 lipids in determining nsCLP. A panel of 3 lipid biomarkers showed great potential for nsCLP diagnosis. FA (20:4) and LPC (18:0) were also significantly downregulated in early serum samples from the nsCLP group in the additional validation cohort. We demonstrate the applicability and robustness of a machine-learning algorithm to analyze lipidomic data for efficient and reliable biomarker screening.
Authors
Shanshan Jia, Weidong Xie, Chunqing Yang, Yizhang Dong, Wenting Luo, Hui Gu, Xiaowei Wei, Wei Ma, Dan Liu, Songying Cao, Yuzuo Bai, Wei Li, Zhengwei Yuan
Sarah E. Glass, Matthew E. Bechard, Zheng Cao, Radhika Aramandla, Ping Zhao, Samuel T. Ellis, Emily H. Green, Elizabeth G. Fisher, Ryan T. Smith, Chelsie K. Sievers, Maria Johnson Irudayam, Frank Revetta, M. Kay Washington, Gregory D. Ayers, Cody N. Heiser, Alan J. Simmons, Yanwen Xu, Yu Wang, Annika Windon, Martha J. Shrubsole, Nicholas O. Markham, Qi Liu, Ken S. Lau, Robert J. CoffeySarah E. Glass, Matthew E. Bechard, Zheng Cao, Radhika Aramandla, Ping Zhao, Samuel T. Ellis, Emily H. Green, Elizabeth G. Fisher, Ryan T. Smith, Chelsie K. Sievers, Maria Johnson Irudayam, Frank Revetta, M. Kay Washington, Gregory D. Ayers, Cody N. Heiser, Alan J. Simmons, Yanwen Xu, Yu Wang, Annika Windon, Martha J. Shrubsole, Nicholas O. Markham, Qi Liu, Ken S. Lau, Robert J. Coffey×Abstract
Dipeptidase-1 (DPEP1) is highly upregulated in colorectal cancer (CRC), with its enzymatic function linked to invasion and metastasis. More recently, DPEP1 was found to serve as a receptor for neutrophils when expressed by activated endothelial cells. It is unknown whether neutrophils bind to DPEP1-expressing CRC cells and whether this impacts features of CRC. Neutrophils have been shown to be tumor promoting in cancers including CRC, where they act to exclude CD8+ T cells. Herein, we show that neutrophils bind DPEP1-expressing CRC cells. In addition, DPEP1 is preferentially expressed in microsatellite-stable (MSS) CRCs, in which there are a paucity of CD8+ T cells, whereas DPEP1 is negatively correlated with microsatellite-unstable (MSI-H) CRCs, which are T cell rich and are more responsive to immunotherapy. Remarkably, carcinogen-treated Dpep1-null mice develop multiple, large, plaque-like, locally invasive adenocarcinomas and squamous cell cancers in the distal colon. These adenocarcinomas exhibit a marked reduction in neutrophils and an influx CD8+ T cells, along with reduced expression of mismatch repair proteins, consistent with features of MSI-H CRC. These results establish DPEP1’s importance in maintaining MSS CRC and its ability to shape the tumor microenvironment.
Authors
Sarah E. Glass, Matthew E. Bechard, Zheng Cao, Radhika Aramandla, Ping Zhao, Samuel T. Ellis, Emily H. Green, Elizabeth G. Fisher, Ryan T. Smith, Chelsie K. Sievers, Maria Johnson Irudayam, Frank Revetta, M. Kay Washington, Gregory D. Ayers, Cody N. Heiser, Alan J. Simmons, Yanwen Xu, Yu Wang, Annika Windon, Martha J. Shrubsole, Nicholas O. Markham, Qi Liu, Ken S. Lau, Robert J. Coffey
×Abstract
The inflammatory response after myocardial infarction (MI) is a precisely regulated process that greatly affects subsequent wound healing and remodeling. However, understanding about the process is still limited. Macrophages are critically involved in inflammation resolution after MI. Krüppel-like factor 9 (Klf9) is a C2H2 zinc finger–containing transcription factor that has been implicated in glucocorticoid regulation of macrophages. However, the contribution of Klf9 to macrophage phenotype and function in the context of MI remains unclear. Our study revealed that KLF9 deficiency resulted in higher mortality and cardiac rupture rate, as well as a considerable exacerbation in cardiac function. Single-cell RNA sequencing and flow cytometry analyses revealed that, compared with WT mice, Klf9–/– mice displayed excessive neutrophil infiltration, insufficient macrophage infiltration, and a reduced proportion of monocyte-derived CD206+ macrophages after MI. Moreover, the expression of IFN-γ/STAT1 pathway genes in Klf9–/– cardiac macrophages was dysregulated, characterized by insufficient expression at 1 day post-MI and excessive expression at day 3 post-MI. Mechanistically, Klf9 directly binds to the promoters of Stat1 gene, regulating its transcription. Overall, these findings indicate that Klf9 beneficially influences wound healing after MI by modulating macrophage recruitment and differentiation by regulating the IFN-γ/STAT1 signaling pathway.
Authors
Sheng Xu, Hao Li, Jun Han, Yawei Xu, Niannian Li, Wenliang Che, Feng Liu, Wenhui Yue
Usha S. Polaki, Trey E. Gilpin, Apoorva T. Patil, Emily Chiu, Ruth Baker, Peng Liu, Tatiana S. Pavletich, Morteza Seifi, Paula M. Mañán-Mejías, Jordan Morrissey, Jenna Port, Rene Welch Schwartz, Irene M. Ong, Dina El-Rayes, Mahmoud A. Khalifa, Pei Hui, Vanessa L. Horner, María Virumbrales-Muñoz, Britt K. Erickson, Lisa Barroilhet, Stephanie M. McGregor, Emery H. Bresnick, Daniel R. MatsonUsha S. Polaki, Trey E. Gilpin, Apoorva T. Patil, Emily Chiu, Ruth Baker, Peng Liu, Tatiana S. Pavletich, Morteza Seifi, Paula M. Mañán-Mejías, Jordan Morrissey, Jenna Port, Rene Welch Schwartz, Irene M. Ong, Dina El-Rayes, Mahmoud A. Khalifa, Pei Hui, Vanessa L. Horner, María Virumbrales-Muñoz, Britt K. Erickson, Lisa Barroilhet, Stephanie M. McGregor, Emery H. Bresnick, Daniel R. Matson×Abstract
BACKGROUND A priori knowledge of recurrence risk in patients with nonmetastatic (International Federation of Gynecology and Obstetrics [FIGO] stage I) uterine serous carcinoma (USC) would enable a risk-stratified approach to the use of adjuvant chemotherapy. This would greatly reduce treatment-related morbidity and be predicted to improve survival.METHODS GATA2 expression was scored by IHC across a retrospective multiinstitutional cohort of 195 primary USCs. Associations between GATA2 levels and clinicopathologic metrics were evaluated using Student’s t test, Fisher’s exact test, Kaplan-Meier method, and Cox proportional hazard ratio. Invasion in patient-derived USC cells was assessed by Student’s t test. RNA-Seq, anti-GATA2 ChIP-Seq, and confirmatory Western blotting enabled identification of GATA2 targets.RESULTS Patients with FIGO stage I GATA2hi USCs had 100% recurrence-free and 100% cancer-related survival, which was significantly better than patients with GATA2lo USCs. In patients for whom adjuvant chemotherapy was omitted, patients with GATA2hi USC had 100% recurrence-free 5-year survival compared with 60% recurrence-free survival in patients with GATA2lo USC. Depletion of GATA2 in patient-derived USC cells increased invasion in vitro.CONCLUSION Routine GATA2 IHC identifies 33% of patients with FIGO stage I USC who have a greatly reduced risk of posthysterectomy USC recurrence. Our results suggest that a GATA2-guided personalized medicine approach could be rapidly implemented in most hospital settings, would reduce treatment-related morbidity, and would likely improve outcomes in patients with USC.FUNDING NIH grants R01 DK068634, P30 CA014520, S10 OD023526, K08 DK127244, T32 HL007899, the UW-Madison Department of Pathology and Laboratory Medicine, the UW-Madison Centennial Scholars Program, the Diane Lindstrom Foundation, the American Cancer Society, the V Foundation, The Hartwell Foundation, and the UMN Department of Obstetrics, Gynecology, and Women’s Health.
Authors
Usha S. Polaki, Trey E. Gilpin, Apoorva T. Patil, Emily Chiu, Ruth Baker, Peng Liu, Tatiana S. Pavletich, Morteza Seifi, Paula M. Mañán-Mejías, Jordan Morrissey, Jenna Port, Rene Welch Schwartz, Irene M. Ong, Dina El-Rayes, Mahmoud A. Khalifa, Pei Hui, Vanessa L. Horner, María Virumbrales-Muñoz, Britt K. Erickson, Lisa Barroilhet, Stephanie M. McGregor, Emery H. Bresnick, Daniel R. Matson
×Abstract
Metastatic outgrowth in distant microscopic niches requires sufficient nutrients, including fatty acids (FAs), to support tumor growth and to generate an immunosuppressive tumor microenvironment (TME). However, despite the important role of FAs in metastasis, the regulation of FA supply in metastatic niches has not been defined. In this report, we show that tumor endothelium actively promotes outgrowth and restricts antitumor cytolysis by transferring FAs into developing metastatic tumors. We describe a process of transendothelial FA delivery via endosomes that requires mTORC1 activity. Thus, endothelial cell–specific targeted deletion of Raptor (RptorECKO), a unique component of the mTORC1 complex, significantly reduced metastatic tumor burden that was associated with improved markers of T cell cytotoxicity. Low-dose everolimus that selectively inhibited endothelial mTORC1 improves immune checkpoint responses in metastatic disease models. This work reveals the importance of transendothelial nutrient delivery to the TME, highlighting a future target for therapeutic development.
Authors
Deanna N. Edwards, Shan Wang, Kelby Kane, Wenqiang Song, Laura C. Kim, Verra M. Ngwa, Yoonha Hwang, Kevin Ess, Mark R. Boothby, Jin Chen
×Abstract
Multiple members of the caveolae-associated protein (Cavin) family are implicated in angiogenesis. However, the specific role of CAVIN3 in pathological angiogenesis within the eye remains unclear. The present study demonstrated that CAVIN3 knockdown in endothelial cells (ECs) promoted vascular normalization in ocular pathological neovascularization. Elevated CAVIN3 expression was observed in the ECs of retinal pigment epithelium/choroid complexes from patients with neovascular age-related macular degeneration and fibrovascular membranes from patients with proliferative diabetic retinopathy. Additionally, upregulated Cavin3 expression was detected in laser-induced choroidal neovascularization (CNV) and oxygen-induced retinopathy (OIR) mouse models. In both OIR and CNV mice, Cavin3 knockdown inhibited pathological neovascularization. Cavin3 deficiency further disrupted EC proliferation and vascular sprouting, thereby promoting vascular normalization by partially restoring microenvironmental hypoxia and reestablishing pericyte-EC interactions. Mechanistically, we demonstrated that zinc finger E-box–binding homeobox 1 (ZEB1) regulated CAVIN3 transcription in ECs under hypoxic conditions. CAVIN3 deficiency modulated pathological vascularization by inhibiting ERK phosphorylation, which downregulated jagged 1 (JAG1) expression. Conclusively, this study elucidated the protective role of endothelial CAVIN3 deficiency in pathological neovascularization models, addressing a gap in understanding the regulatory role of Cavins in angiogenesis. These findings suggested a therapeutic direction for ocular neovascular diseases.
Authors
Weiqi Li, Yeran Zhang, Hongjing Zhu, Na Su, Ruxu Sun, Xiying Mao, Qin Yang, Songtao Yuan
Youchen Yan, Tingting Zhang, Xin He, Tailai Du, Gang Dai, Xingfeng Xu, Zhuohui Chen, Jialing Wu, Huimin Zhou, Yazhi Peng, Yan Li, Chen Liu, Xinxue Liao, Yugang Dong, Jing-song Ou, Zhan-Peng HuangYouchen Yan, Tingting Zhang, Xin He, Tailai Du, Gang Dai, Xingfeng Xu, Zhuohui Chen, Jialing Wu, Huimin Zhou, Yazhi Peng, Yan Li, Chen Liu, Xinxue Liao, Yugang Dong, Jing-song Ou, Zhan-Peng Huang×Abstract
Inflammation is a critical pathological process in myocardial infarction. Although immunosuppressive therapies can mitigate inflammatory responses and improve outcomes in myocardial infarction, they also increase the risk of infections. Identifying novel regulators of local cardiac inflammation could provide safer therapeutic targets for myocardial ischemia/reperfusion injury. In this study, we identified a previously uncharacterized micropeptide, which we named Inflammation Associated MicroPeptide (IAMP). IAMP is predominantly expressed in cardiac fibroblasts, and its expression is closely associated with cardiac inflammation. Downregulation of IAMP promotes, whereas its overexpression prevents, the transformation of cardiac fibroblasts into a more inflammatory phenotype under stressed/stimulated conditions, as evidenced by changes in the expression and secretion of proinflammatory cytokines. Consequently, loss of IAMP function leads to uncontrolled inflammation and worsens cardiac injury following ischemia/reperfusion surgery. Mechanistically, IAMP promotes the degradation of HIF-1α by interacting with its stabilizing partner HSP90 and, thus, suppresses the transcription of proinflammatory genes downstream of HIF-1α. This study underscores the significance of fibroblast-mediated inflammation in cardiac ischemia/reperfusion injury and highlights the therapeutic potential of targeting micropeptides for myocardial infarction.
Authors
Youchen Yan, Tingting Zhang, Xin He, Tailai Du, Gang Dai, Xingfeng Xu, Zhuohui Chen, Jialing Wu, Huimin Zhou, Yazhi Peng, Yan Li, Chen Liu, Xinxue Liao, Yugang Dong, Jing-song Ou, Zhan-Peng Huang
Michael H. Lee, Thaís C. F. Menezes, Julie A. Reisz, Francesca I. Cendali, Eloara V. M. Ferreira, Jaquelina S. Ota-Arakaki, Priscila A. Sperandio, Rahul Kumar, Claudia Mickael, Martin M. Ieong, Juliana Lucena Santos, Ana Carolina B. Duarte, Dara C. Fonseca Balladares, Kevin Nolan, Rubin M. Tuder, Paul M. Hassoun, Angelo D’Alessandro, Rudolf K. F. Oliveira, Brian B. GrahamMichael H. Lee, Thaís C. F. Menezes, Julie A. Reisz, Francesca I. Cendali, Eloara V. M. Ferreira, Jaquelina S. Ota-Arakaki, Priscila A. Sperandio, Rahul Kumar, Claudia Mickael, Martin M. Ieong, Juliana Lucena Santos, Ana Carolina B. Duarte, Dara C. Fonseca Balladares, Kevin Nolan, Rubin M. Tuder, Paul M. Hassoun, Angelo D’Alessandro, Rudolf K. F. Oliveira, Brian B. Graham×Abstract
Pathologic implications of dysregulated pulmonary vascular metabolism to pulmonary arterial hypertension (PAH) are increasingly recognized, but their clinical applications have been limited. We hypothesized that metabolite quantification across the pulmonary vascular bed in connective tissue disease–associated (CTD-associated) PAH would identify transpulmonary gradients of pathobiologically relevant metabolites, in an exercise stage–specific manner. Sixty-three CTD patients with established or suspected PAH underwent exercise right heart catheterization. Using mass spectrometry–based metabolomics, metabolites were quantified in plasma samples simultaneously collected from the pulmonary and radial arteries at baseline and during resistance-free wheeling, peak exercise, and recovery. We identified uptake and excretion of metabolites across the pulmonary vascular bed, unique and distinct from single vascular site analysis. We demonstrated the physiological relevance of metabolites previously shown to promote disease in animal models and end-stage human lung tissues, including acylcarnitines, glycolytic intermediates, and tryptophan catabolites. Notably, pulmonary vascular metabolite handling was exercise stage specific. Transpulmonary metabolite gradients correlated with hemodynamic endpoints largely during free-wheeling. Glycolytic intermediates demonstrated physiologic significance at peak exercise, including net uptake of lactate in those with more advanced disease. Contribution of pulmonary vascular metabolism to CTD-PAH pathogenesis and therapeutic candidacy of metabolism modulation must be considered in the context of physiologic stress.
Authors
Michael H. Lee, Thaís C. F. Menezes, Julie A. Reisz, Francesca I. Cendali, Eloara V. M. Ferreira, Jaquelina S. Ota-Arakaki, Priscila A. Sperandio, Rahul Kumar, Claudia Mickael, Martin M. Ieong, Juliana Lucena Santos, Ana Carolina B. Duarte, Dara C. Fonseca Balladares, Kevin Nolan, Rubin M. Tuder, Paul M. Hassoun, Angelo D’Alessandro, Rudolf K. F. Oliveira, Brian B. Graham
Jacqueline H. Starrett, Clara Lemoine, Matthias Guillo, Chantal Fayad, Nabil Kaci, Melissa Neal, Emily A. Pettitt, Melissandre Pache, Qing Ye, My Chouinard, Eric L. Allen, Geneviève Baujat, Robert L. Hudkins, Michael B. Bober, Todd Harris, Ronald V. Swanson, Laurence Legeai-MalletJacqueline H. Starrett, Clara Lemoine, Matthias Guillo, Chantal Fayad, Nabil Kaci, Melissa Neal, Emily A. Pettitt, Melissandre Pache, Qing Ye, My Chouinard, Eric L. Allen, Geneviève Baujat, Robert L. Hudkins, Michael B. Bober, Todd Harris, Ronald V. Swanson, Laurence Legeai-Mallet×Abstract
Achondroplasia (ACH) and hypochondroplasia (HCH), the two most common types of dwarfism, are each caused by FGFR3 gain-of-function mutations that result in increased FGFR3 signaling, which disrupts chondrogenesis and osteogenesis, resulting in disproportionately shortened long bones. In this study, TYRA-300, a potent and selective FGFR3 inhibitor, was evaluated in 3 genetic contexts: wild-type mice, the Fgfr3Y367C/+ mouse model of ACH, and the Fgfr3N534K/+ mouse model of HCH. In each model, TYRA-300 treatment increased nasoanal length and tibia and femur length. In the two FGFR3-altered models, TYRA-300–induced growth partially restored the disproportionality of long bones. Histologic analysis of the growth plate in Fgfr3Y367C/+ mice revealed that TYRA-300 mechanistically increased both proliferation and differentiation of chondrocytes. Importantly, children with ACH can experience medical complications due to foramen magnum stenosis, and TYRA-300 significantly improved the size and shape of the skull and foramen magnum in Fgfr3Y367C/+ mice. Spinal stenosis is also a frequent complication, and TYRA-300 increased the lumbar vertebrae length and improved the shape of the intervertebral discs in both models. Taken together, these studies demonstrate that the selective FGFR3 inhibitor TYRA-300 led to a significant increase in bone growth in two independent FGFR3-driven preclinical models as well as in wild-type mice.
Authors
Jacqueline H. Starrett, Clara Lemoine, Matthias Guillo, Chantal Fayad, Nabil Kaci, Melissa Neal, Emily A. Pettitt, Melissandre Pache, Qing Ye, My Chouinard, Eric L. Allen, Geneviève Baujat, Robert L. Hudkins, Michael B. Bober, Todd Harris, Ronald V. Swanson, Laurence Legeai-Mallet
×Abstract
Patients with viral myocarditis (VMC) exhibit evident autonomic nervous system imbalance, and adverse cardiac remodeling is involved in impaired cholinergic function. The α7 nicotinic acetylcholine receptor (α7nAChR), which is a neurotransmitter receptor, exerts immunoregulatory effects. Recent advances have illuminated the evolution and functions of peripheral and cardiac B cells in heart disease. However, the role of α7nAChR expressed by B cells in the progression of VMC has not been established. We revealed the neuroimmune communication landscape in the heart and found that the phenotypes of cardiac and splenic B cells and their α7nAChR expression changed dynamically during the progression of VMC to dilated cardiomyopathy. α7nAChR on B cells serves as a negative regulator by inhibiting their proinflammatory functions and signaling pathways. B cell–specific α7nAChR deficiency exacerbated myocardial inflammation, fibrosis, and cardiac dysfunction. However, these effects were abrogated in non-B cells from mice with IL-17A knockdown. Enhanced degradation of acetylcholine leads to an imbalance in cholinergic signaling, resulting in impaired neurotransmission. The acetylcholinesterase inhibitor pyridostigmine bromide could improve cardiac remodeling and prevent the progression of VMC to the chronic phase, which was partly dependent on the α7nAChR on B cells. Our findings provide notable insights into cardiac-neural-immune communication during myocardial injury.
Authors
Jing Lu, Keren Chen, Zhihong Cen, Yanlan Huang, Yong Li, LiLi Chen, Weifeng Wu
×Abstract
Urinary tract infections (UTIs) are one of the most commonly encountered infections in clinical practice, in which psychological stress is a critical pathological contributor to modulate immune function. However, mechanistic pathways linking stress networks in the brain to bladder infection remain poorly understood. In this study, we discovered that acute stress treatment suppressed bladder inflammation in mice with UTIs, and a substantial number of neurons showing overlap between inflammation-associated markers and retrograde labeling were observed in the paraventricular nucleus (PVN) brain region of these mice. Activation of the PVN alleviated uropathogenic Escherichia coli–induced bladder inflammatory response. Moreover, a blocked hypothalamic-pituitary-adrenal axis reversed the antiinflammatory reflex mediated by acute stress, suggesting that glucocorticoids may modulate UTIs through the brain-body circuit. Single-cell RNA-Seq of bladder immune cells revealed that type 2 innate lymphoid (ILC2) cells expressed abundant levels of glucocorticoid receptor. The activation of the PVN effectively inhibited the expression of the pro-inflammatory cytokine colony-stimulating factor 2 by ILC2 cells through direct regulation of cell-intrinsic glucocorticoid signaling. Ultimately, our study has implications for the positioning of the brain-body circuit for UTI treatment.
Authors
Yaxiao Liu, Jinhua Wang, Junyang Lin, Dingqi Sun, Kejia Zhu, Tongxiang Diao, Qiang Fu, Qingyu Ren
×Abstract
Severe asthma in children is notoriously difficult to treat, and its immunopathogenesis is complex. In particular, the contribution of T cells and relationships to antiviral immunity remain enigmatic. Here, we coupled deep phenotyping with machine learning methods to elucidate the dynamics of T cells in the lower airways of children with treatment-refractory recurrent wheeze, and examine rhinovirus (RV) as a driver. Our strategy revealed a T cell landscape dominated by type 1 and type 17 CD8+ signatures. Interrogation of phenotypic relationships coupled with trajectory mapping identified T cell migratory and differentiation pathways spanning the blood and airways that culminated in tissue residency, and involved transitions between type 1 and type 17 tissue-resident types. These dynamics were reflected in cytokine polyfunctionality. Use of machine learning tools to cross-compare T cell populations that were enriched in the airways of RV-positive children with those induced in the blood following experimental RV challenge precisely pinpointed RV-responsive signatures that contributed to T cell migratory and differentiation pathways. Despite their rarity, these signatures were also detected in the airways of RV-negative children. Together, our results underscore the aberrant nature of type 1 immunity in the airways of children with recurrent wheeze, and implicate an important viral trigger as a driver.
Authors
Naomi Bryant, Lyndsey M. Muehling, Kristin Wavell, W. Gerald Teague, Judith A. Woodfolk
×Abstract
BACKGROUND The graft-versus-leukemia (GVL) effect contributes to the efficacy of allogeneic stem cell transplantation (alloSCT). However, relapse, indicative of GVL failure, is the greatest single cause of treatment failure. Based on preclinical data showing that IFN-γ is important to sensitize myeloblasts to alloreactive T cells, we performed a phase I trial of IFN-γ combined with donor leukocyte infusions (DLIs) in myeloblastic malignancies that relapsed after HLA-matched alloSCT.METHODS Patients with relapsed acute myeloid leukemia or myelodysplastic syndrome after alloSCT were eligible. Patients self-administered IFN-γ for 4 weeks (cohort 1) or 1 week (cohort 2), followed by DLI and concurrent IFN-γ for a total of 12 weeks. Bone marrow samples were analyzed by single-cell RNA sequencing (scRNA-Seq) to assess in vivo responses to IFN-γ by malignant myeloblasts.RESULTS IFN-γ monotherapy was well tolerated by all participants (n = 7). Treatment-related toxicities after DLI included grade I–II graft-versus-host disease (n = 5), immune effector cell–associated neurotoxicity syndrome (n = 2), and idiopathic pulmonary syndrome (n = 1), all of which resolved with corticosteroids. Four of 6 DLI recipients achieved minimal residual disease-negative complete remissions and full donor hematopoietic recovery. Median overall survival was 579 days (range, 97–906) in responders. scRNA-Seq validated in vivo activation of the IFN-γ response pathway in hematopoietic stem cell–like or myeloid progenitor cells after IFN-γ in analyzed samples.CONCLUSION IFN-γ was safe and well tolerated in this phase I study of IFN-γ for relapsed acute myeloid leukemia and myelodysplastic syndrome after alloSCT, with a promising efficacy signal when combined with DLI. Larger studies are needed to formally test the efficacy of this approach.TRIAL REGISTRATION ClinicalTrials.gov NCT04628338.FUNDING UPMC Hillman Cancer Center Cancer Immunology and Immunotherapy Program Pilot Award and Cure Within Reach: Drug Repurposing Clinical Trials to Impact Blood Cancers.
Authors
Sawa Ito, Emily Geramita, Kedwin Ventura, Biswas Neupane, Shruti Bhise, Erika M. Moore, Scott Furlan, Warren D. Shlomchik
Mark Rusznak, Christopher M. Thomas, Jian Zhang, Shinji Toki, Weisong Zhou, Masako Abney, Danielle M. Yanda, Allison E. Norlander, Craig A. Hodges, Dawn C. Newcomb, Mark H. Kaplan, R. Stokes Peebles Jr., Daniel P. CookMark Rusznak, Christopher M. Thomas, Jian Zhang, Shinji Toki, Weisong Zhou, Masako Abney, Danielle M. Yanda, Allison E. Norlander, Craig A. Hodges, Dawn C. Newcomb, Mark H. Kaplan, R. Stokes Peebles Jr., Daniel P. Cook×Abstract
Type 2 inflammatory diseases, including asthma, sinusitis, and allergic bronchopulmonary aspergillosis, are common in cystic fibrosis (CF). CD4+ Th2 cells promote these diseases through secretion of IL-4, IL-5, and IL-13. Whether the CF transmembrane conductance regulator (CFTR), the mutated protein in CF, has a direct effect on Th2 development is unknown. Using murine models of CFTR deficiency and human CD4+ T cells, we show that CD4+ T cells expressed Cftr transcript and CFTR protein following activation. Loss of T cell CFTR expression increased Th2 cytokine production compared with control cells. Mice with CFTR-deficient T cells developed increased allergic airway disease to Alternaria alternata extract compared with control mice. Culture of CFTR-deficient Th2 cells demonstrated increased IL-4Rα expression and increased sensitivity to IL-4 with greater induction of GATA3 and IL-13 compared with control Th2 cell cultures. The CFTR potentiator ivacaftor reduced allergic inflammation and type 2 cytokine secretion in bronchoalveolar lavage of humanized CFTR mice following Alternaria alternata extract challenge and decreased Th2 development in human T cell culture. These data support a direct role of CFTR in regulating T cell sensitivity to IL-4 and demonstrate a potential CFTR-specific therapeutic strategy for Th2 cell–mediated allergic disease.
Authors
Mark Rusznak, Christopher M. Thomas, Jian Zhang, Shinji Toki, Weisong Zhou, Masako Abney, Danielle M. Yanda, Allison E. Norlander, Craig A. Hodges, Dawn C. Newcomb, Mark H. Kaplan, R. Stokes Peebles Jr., Daniel P. Cook