Research ArticleImmunologyInfectious disease
Open Access |  10.1172/jci.insight.183076
10.1172/jci.insight.183076
Phenotype and function of IL-10–producing NK cells in individuals with malaria experience
Sarah A. McNitt,1 Jenna K. Dick,2,3 Maria Andrea Hernandez-Castaneda,2,3 Jules Sangala,2,3 Mark Pierson,3,4 Marissa Macchietto,5 Kristina S. Burrack,3,6 Peter D. Crompton,7 Karl Seydel,1,8 Sara E. Hamilton,3,4 and Geoffrey T. Hart2,3
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by McNitt, S. in: PubMed | Google Scholar
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by Dick, J. in: PubMed | Google Scholar
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by Hernandez-Castaneda, M. in: PubMed | Google Scholar
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by Sangala, J. in: PubMed | Google Scholar
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by Pierson, M. in: PubMed | Google Scholar
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by Macchietto, M. in: PubMed | Google Scholar
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by Burrack, K. in: PubMed | Google Scholar
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by
Crompton, P.
in:
PubMed
|
Google Scholar
|

1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by
Seydel, K.
in:
PubMed
|
Google Scholar
|

1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by Hamilton, S. in: PubMed | Google Scholar
1Department of Osteopathic Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
2Division of Infectious Disease and Internal Medicine, Department of Medicine,
3Center for Immunology,
4Department of Laboratory Medicine and Pathology, and
5Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, Minnesota, USA.
6Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
7Malaria Infection Biology and Immunity Section, Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), NIH, Rockville, Maryland, USA.
8Blantyre Malaria Project, Kamuzu University of Health Sciences, Blantyre, Malawi.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Find articles by Hart, G. in: PubMed | Google Scholar
Authorship note: SAM and JKD contributed equally to this work and are co-first authors.
Published May 8, 2025 - More info
JCI Insight. 2025;10(9):e183076. https://doi.org/10.1172/jci.insight.183076.
© 2025 McNitt et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
P.falciparum infection can trigger high levels of inflammation that lead to fever and sometimes severe disease. People living in malaria-endemic areas gradually develop resistance to symptomatic malaria and control both parasite numbers and the inflammatory response. We previously found that adaptive NK cells correlated with reduced parasite load and protection from symptoms. We also found that murine NK cell production of IL-10 protected mice from experimental cerebral malaria. Human NK cells can also secrete IL-10, but it is unknown what NK cell subsets produce IL-10 or if this is affected by malaria experience. We hypothesized that NK cell immunoregulation may lower inflammation and reduce fever induction. Here, we showed that NK cells from participants with malaria experience make significantly more IL-10 than participants with no malaria experience. We then determined the proportions of NK cells that are cytotoxic and produce IFN-γ and/or IL-10 and identified a signature of adaptive and checkpoint molecules on IL-10–producing NK cells. Lastly, we found that coculture with primary monocytes, Plasmodium-infected RBCs, and antibody induced IL-10 production by NK cells. These data suggest that NK cells may contribute to protection from malaria symptoms via IL-10 production.
-
Introduction
Malaria is a mosquito-borne infectious disease that claims roughly 600,000 lives per year and is caused by Plasmodium parasites (1). Over 90% of the world’s malaria deaths occur among children living in sub-Saharan Africa. In humans, Plasmodium infection starts by mosquitoes taking a blood meal and injecting sporozoites into the skin whereupon the sporozoites traffic to the liver. After 7 days, infected hepatocytes rupture, releasing thousands of merozoites that start the erythrocytic stage by infecting RBCs. Lastly, some parasites transform into gametocytes; this is the sexual form of Plasmodium that can transmit the parasite back to mosquitoes with a subsequent blood meal. Broadly, this process is characterized into 3 stages: the sporozoite (liver), erythrocytic (blood), and gametocyte (sexual) stages (2). Recent headway has been made with malaria sporozoite stage vaccines and monoclonal antibody development (3–5). These approaches target the circumsporozoite protein (CSP) to block infection progression or reduce the infectious dose of merozoites released from the liver. However, for people who progress to the erythrocytic stage of infection, where CSP is not expressed, susceptibility may remain to the symptomatic and severe manifestations of malaria, including cerebral malaria and severe malaria anemia.
During the erythrocytic stage of infection, the optimal host immune response maintains a delicate balance between curbing parasite growth and minimizing host immunopathology. Proposed mechanisms to kill Plasmodium-infected RBCs and limit parasite growth are thought to include phagocytosis and direct cytotoxic killing of infected RBCs (6–12). Recently, NK cells have been shown to inhibit P.falciparum growth in vitro through antibody-dependent cellular cytotoxicity (ADCC) (6). Not all subsets of NK cells are equally adept at ADCC. Previous work showed that trained subsets of NK cells, originally defined in the context of cytomegalovirus (CMV) infection and referred to as adaptive NK cells, display memory-like functions and enhanced ADCC capabilities (13–17). Characteristic phenotypes associated with adaptive NK cells are increased expression of NKG2C and CD57, which are activation and maturation markers, respectively (16, 17), and reduced expression of the signaling adapter Fc receptor γ chain (Fcer1g) and transcription factor promyelocytic leukemia zinc-finger protein (PLZF) (14, 18). We and others have found that individuals with malaria experience have an adaptive NK cell subset that lacks Fcer1g and PLZF and correlates with reduced parasitemia and protection from malaria symptoms (7, 8).
The adaptive NK cell subset identified in individuals with malaria experience was also enhanced for both cytotoxic and inflammatory cytokine production in vitro (7, 8). This finding, combined with data showing that NK cell ADCC inhibits the growth of Plasmodium falciparum in vitro, supports the hypothesis that this adaptive NK cell subset contributes to reducing parasite load in vivo. Specifically, when parasite load is low, the development of malaria symptoms does not directly correlate with parasite load, as many individuals maintain substantial parasite burden while remaining asymptomatic (11, 19, 20). Considering IFN-γ, a strongly proinflammatory cytokine, has been shown to be produced by adaptive NK cells, it is somewhat surprising that their increased proportion correlates with reduced symptoms (e.g., fever), given that inflammation is a driver of fever. Therefore, we explored the hypothesis that the role of NK cells during malaria infection may extend beyond direct parasite control and include antiinflammatory mechanisms that prevent symptomatic malaria.
In malaria-naive individuals, P.falciparum infection leads to robust production of inflammatory cytokines such as TNF-α, IL-1β, IL-12, IL-6, and IFN-γ and subsequent development of fever. Controlling this response is critical to prevent aberrant inflammation and damage to host tissues (21–23). As an important component of this regulation, IL-10 suppresses inflammation during malaria and other infections by dampening the production of proinflammatory cytokines, downregulating MHC-II on antigen presenting cells (24), and promoting humoral immunity (25–27). IL-10 is made by many cell types but is most associated with CD4+ T cells (28–32). Systemic cytokines are detected in both nonsevere and severe malaria, and the ratio of IL-10 levels to inflammatory cytokines can be an indicator of disease (33). We and others have shown NK cells can make IL-10, but it is unknown how much systemic IL-10 is derived from NK cells and what role NK cell–produced IL-10 plays during malaria infection.
Our data from the experimental cerebral malaria (ECM) mouse model show that NK cells can be induced to produce IL-10, which protected mice from severe disease (34). Additionally, we found that secretion of IL-10 by human malaria-naive NK cells is induced in vitro by cytokines IL-15, IL-21, and IL-12. Cytokines IL-15 and IL-21 are key promotors of NK cell survival and proliferation, while IL-12 is an effector cytokine produced by myeloid cells that can stimulate NK cell activation. The IL-10 made by human NK cells was detected via ELISA and real-time PCR, but the proportion and phenotype of NK cells that produce IL-10 was unknown.
In this study, we analyzed blood from adolescents and young adults from Mali, Africa — a malaria endemic country — who have, by this age, experienced multiple exposures to P.falciparum. Some of these individuals still develop symptomatic malaria infection, which is defined by having a fever (>37.5°C) and the presence of parasites in the blood (>2,500 parasites/μL). We also analyzed samples from individuals living in the United States with no previous exposure to Plasmodium species. We found that individuals with malaria experience had a significantly higher proportion of NK cells producing IL-10 relative to malaria-naive individuals. Some NK cells were enriched for cytolytic activity, IFN-γ, and/or IL-10 production, but others performed multiple functions. IL-10–producing NK cells expressed a unique adaptive and checkpoint marker signature. Lastly, when NK cells were incubated with P.falciparum–infected RBCs in the presence of monocytes and anti-Plasmodium antibodies, the NK cells were induced to make IL-10. This data set demonstrates that IL-10–producing NK cells are more prevalent in individuals living in this malaria endemic area and suggests that human NK cells producing IL-10 may have a protective role in malaria infection.
-
Results
NK cells from individuals in malaria-endemic regions secrete more IL-10 than NK cells from malaria-naive individuals. The observation that NK cells could rescue mice from ECM death in a manner that was dependent on IL-10 secretion led us to hypothesize that IL-10 release from NK cells may also play a protective role in human Plasmodium infection. We sought to determine whether NK cells from individuals who have undergone many seasonal exposures to Plasmodium infections have a higher propensity to secrete IL-10 than those from malaria-naive individuals. To test this hypothesis in vitro, we exposed NK cells from healthy donors who were either naive to malaria (USA) or had a history of malaria (Mali) to inflammatory conditions using a previously published 6-day cytokine stimulation procedure (Figure 1A) (34). We then measured the release of IL-10 using an extracellular IL-10 capture reagent. In line with our previous work showing that human NK cells secrete minimal IL-10 with IL-15 treatment alone (34), we observed that less than 1% of malaria-naive and malaria-experienced NK cells release IL-10 under these conditions (Figure 1, B–D). However, when IL-21 is included during the 6 days of culture, followed by a short incubation with IL-12, we found significantly greater IL-10 secretion from NK cells from individuals naive to malaria (malaria-naive) and those with a history of malaria (malaria-experienced). Surprisingly, about 20-fold more NK cells from malaria-experienced individuals secreted IL-10 as compared with malaria-naive controls (Figure 1, C and D). We also examined individuals with malaria experience that were still susceptible to clinical malaria over the course of the malaria season. This sampling occurred before the start of the malaria season (Pre-Malaria), when the person presented with clinical malaria (Malaria), and 1 week following diagnosis and treatment (Convalescent) (Figure 1E) (11). Overall, there was a relatively stable frequency of NK cells producing IL-10 before, during, and after an individual presented with clinical malaria.
 Figure 1
Figure 1NK cells from malaria-experienced individuals (Mali) secrete more IL-10 than NK cells from individuals naive for malaria (USA). (A) Experimental design for cytokine stimulation. (B) Example flow cytometry staining of IL-10 production by CD56+ NK cells treated with IL-15 alone (left) (control) or with a combination of IL-15 and IL-21 for 6 days followed by IL-12 on Day 7 (right) (Cytokine stim). Malaria-naive (USA) (top) and malaria-experienced (Mali) (bottom) individuals. (C and D) Comparison of IL-10 production from individuals naive for malaria-naive (USA) (C) and malaria-experienced (Mali) (D) individuals. (E) Comparison of IL-10 production from malaria-experienced individuals before the malaria season (Pre-Malaria), when they presented with malaria (Malaria), and 7 days after they were treated for malaria (Convalescent). Red horizonal lines represent median values. ****P < 0.0001 as determined by Wilcoxon signed-rank test (C and D) or 1-way ANOVA with Tukey’s multiple-comparison test (E).
In addition to cytokine stimulation alone, we also assessed IL-10 secretion by NK cells during ADCC and natural cytotoxicity assays in which NK cells become activated through antibody binding to the Fc receptor (FcgRIIIa/CD16a) or through recognition of stress ligands in the absence of human leukocyte antigens (HLA), respectively (natural cytotoxicity) (Figure 2A). Following cytokine stimulation with IL-15 and IL-21, IL-10 release from NK cells was substantially greater from malaria-experienced individuals in both ADCC (~10-fold increase) and natural cytotoxicity assays (~5-fold increase) that occurred in the presence of IL-12 (Figure 2, B–D). Although not significantly different between the functional assays, the ADCC assay trended toward the greatest increase in the frequency of NK cells producing IL-10 (Figure 2, C and D). Similar to previous findings in the cytokine stimulation assay, IL-10 production required preincubation with both IL-21 and IL-15 in the ADCC assay (Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.183076DS1). When comparing across the 3 assays, the ADCC and natural cytotoxicity IL-10 production was not significantly different than in the cytokine stimulation assay (Supplemental Figure 1B). Because NK cell adaptive phenotype and function can be influenced by prior CMV exposure, we assessed IL-10 production in CMV+ and CMV– individuals. Although all individuals with a history of malaria (malaria-experienced individuals) were also CMV+, no difference in NK cell IL-10 secretion was observed in USA participants who were CMV+ or CMV– during cytokine stimulation, ADCC, or natural cytotoxicity (Supplemental Figure 1C). IL-10 production was not different in NK cells sampled from individuals prior to, during, or after malaria in either cytolytic assay (Figure 2, E and F). Taken together, these data indicate that the NK cells from malaria-experienced individuals more robustly secrete IL-10 compared with those from malaria-naive individuals. Among the assays tested, exposure to cytokines IL-15, IL-21, and IL-12 plus the addition of an ADCC stimulus causes NK cells to produce the most IL-10 (Figures 1 and 2, and Supplemental Figure 1B).
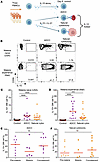 Figure 2
Figure 2NK cells produce IL-10 during antibody-dependent cellular cytotoxicity (ADCC) and natural cytotoxicity. (A) Experimental design for ADCC and natural cytotoxicity assays. Patients from Figure 1 are the same as those in Figure 2 for the control, ADCC, and natural cytotoxicity groups when sufficient cell numbers were available to perform multiple assays. (B) IL-10 production from NK cells treated with IL-15 alone (control) (left), with IL-15 and IL-21 for 6 days followed by IL-12 and either an ADCC (middle) or natural cytotoxicity (right) assay. (C and D) Comparison of ADCC or natural cytotoxicity-induced IL-10 production from the NK cells of malaria-naive (C) or malaria-experienced (D) individuals. (E and F) Comparison of IL-10 production from malaria susceptible individuals before the malaria season (Pre-Malaria), when they got malaria (Malaria), and 7 days after they were treated for malaria (Convalescent) for ADCC (E) or natural cytotoxicity (F). Red horizonal lines represent median values ***P < 0.001 ****P < 0.001 as determined by 1-way ANOVA with Tukey’s multiple-comparison test (C–F). In a Kruskal-Wallis test with Dunn’s post hoc comparisons, the ADCC and natural cytotoxicity groups were not significantly different from the cytokine stimulation group in Figure 1 (Supplemental Figure 1B).
NK cells coproduce IL-10, IFN-γ, and CD107a. From the observations noted above, we postulated that IL-10 may be secreted at the same time as cytotoxic degranulation. When NK cells degranulate their cytolytic granules, the granule membrane fuses with the cell membrane exposing CD107a (LAMP-1) on the surface of the cell. NK cells are also known to produce multiple proinflammatory cytokines, such as IFN-γ, during degranulation. We tested for all 3 molecules (CD107a, IFN-γ, and IL-10) following ADCC and natural cytotoxicity assays as in Figure 2 with the exception that no IL-12 was added (Figure 3A). Also, to assess production of all 3 molecules, we captured IL-10 release, stained intracellularly for IFN-γ, and monitored surface expression of CD107a (gating strategy shown in Supplemental Figure 1D). As expected, IFN-γ was readily produced by NK cells in all conditions (Figure 3A). CD107a was also detected in all assays but was greatest in the natural cytotoxicity assay (Figure 3A). In a pairwise analysis, coexpression of IFN-γ and IL-10 was evident (Figure 3, B–D) as well as coexpression of CD107a and IL-10 (Figure 3, C and D). Using Simplified Presentation of Incredibly Complex Evaluations (SPICE) analysis, we compared the proportions of cells that were expressing CD107a, IFN-γ, and IL-10 as single, double, and triple NK cell producers in all 3 stimulation assays (Figure 3D). Cytokine and ADCC stimulation generated similar patterns of effector molecule expression, with most cells expressing IFN-γ, followed by IL-10 and IFN-γ coexpression. We also observed some triple producers (IFN-γ, IL-10, CD107a) but noted few cells that expressed only IL-10 or CD107a. During natural cytotoxicity, most cells produced both CD107a and IFN-γ. The frequency of cells secreting IL-10 was reduced and was mainly found in conjunction with both IFN-γ and CD107a.
 Figure 3
Figure 3IL-10 is coexpressed with IFN-γ and CD107a. (A) Representative flow cytometry data of IL-10 by IFN-γ (top) or by CD107a (bottom) following IL-15 alone (Control) (left) or IL-15 and IL-21 for 6 days and then with cytokine stimulation (IL-12) (middle left), natural cytotoxicity (No IL-12) (middle right), or ADCC (No IL-12) (right) assay. (B) For individuals with malaria experience, proportions of Boolean gates of IL-10 and IFN-γ for cytokine stimulation (left), ADCC (middle), or natural cytotoxicity (right). (C) For individuals with malaria experience, proportions of Boolean gates of IL-10 and CD107a for ADCC (left) or natural cytotoxicity (right). (D) SPICE analysis of the coexpression of IL-10, IFN-γ, and CD107a following cytokine stimulation, ADCC, or natural cytotoxicity. (E–L) For an ADCC assay, first, cells of each functional group were gated (selected) being IL-10–CD107a+, IL-10+CD107a+, or IL-10+CD107a–. Then the proportion of each NK cell marker was determined. The proportion of each NK cell marker as a percentage of each functional group is shown. Red horizonal lines are median. **P <0.01, ***P < 0.001, ****P <0.0001 by a 1-way ANOVA with Tukey’s multiple-comparison test.
The phenotype of IL-10 secreting NK cells has not been reported. To discover extracellular markers that correlate with IL-10 release in an unbiased manner, we utilized a 350-marker flow cytometry screening panel and single-cell RNA-Seq to analyze cytokine stimulated malaria-naive NK cells. Results from these screens revealed several markers that were preferentially expressed on IL-10+ NK cells compared with IL-10– NK cells (Supplemental Figure 2, A–C). We noted several genes in this dataset — CTLA4, TNFRSF9, PDCD1, LAG3, SIGLEC7, KLRG1, and TIGIT — that have been characterized as immune checkpoint molecules in NK cells (35–40). Given the similarity between IL-10+ NK cells and IL-10+ Tr1 T cells, we also examined several transcription factors associated with IL-10 production in Tr1 cells. We found that IRF4 and ZBED2 were significantly associated with IL-10+ NK cells (Supplemental Figure 2B). We also identified markers less studied in NK cell biology such as CX3CR1, CD45RO, and CRTAM (41, 42). These markers were integrated into the study’s flow cytometry assays as well as markers associated with adaptive NK cells and viral infection, such as NKG2C, FcRγ chain (FCER1G), PLZF (ZBTB16), and CD8 (7, 14, 16–18, 43, 44). Recently, another CD56–, CD7+, FcRγ chain negative NK cell population was found to be associated with malaria exposure and protection from malaria, similar to CD56 dim (positive), FcRγ chain negative NK cells (7, 45). We assessed the proportions of CD56 positive and CD56– (CD7+, CD3–) NK cells before (Day 0) and after 6 days of cytokine incubation (8). We found that, after cell culture, the CD56– NK cell proportion was drastically lowered indicating either death or conversion to a CD56+ phenotype (Supplemental Figure 2D). Therefore, we chose to assess CD56+ NK cells for expression of IL-10 secretion from malaria-naive and malaria-experienced individuals.
In the ADCC assay, we found 3 populations of cells: those that are solely producing IL-10 and are not cytotoxic (IL-10+, CD107a–), those that are only cytotoxic (IL-10–, CD107a+), and those that coexpress IL-10 and CD107a (IL-10+, CD107a+). Furthermore, because IL-12 was not added to this assay, we conclude that the addition of IL-12 is not required for either IL-10 or IFN-γ production during the ADCC assay. Incorporating the markers we identified in the RNA-Seq and protein screens, we associated markers that were enriched in these functional groups. Siglec-7– and CD45RO+ NK cells were enriched in the IL-10 producers (both IL-10+CD107a– and IL-10+CD107a+ NK cells) (Figure 3, E and F). TIGIT+ NK cells were enriched in the cytotoxic NK cells (both IL-10–CD107a+ and IL-10+CD107a+ NK cells) (Figure 3G). Lastly, 4-1BB+, CTLA-4+, CX3CR1+, NKG2C+, and LAG-3+ NK cells were enriched in the dually cytotoxic and IL-10–producing NK cells (Figure 3, I–L). LAG-3, TIGIT, and TIM-3 are also expressed on Tr1 cells following Plasmodium infection (46).
We also noted that stimulation in ADCC or natural cytotoxicity assays directly ex vivo (Day 0) induces IFN-γ production in 5%–20% of the NK cells (Supplemental Figure 3, A and B), whereas there is very little IL-10 made. Instead, it took cytokine stimulation to induce the NK cells to produce IL-10 (Day 6) (Supplemental Figure 3, A and B). This is a similar trend to reports of NK cell IFN-γ and IL-10 production in vivo, which indicate that IL-10 initiates after an early IFN-γ response to infection (34, 47).
NK cells secreting IL-10 express adaptive and immune checkpoint molecules. We found that PD-1+, 4-1BB+, LAG-3+, KLRG1–, PLZF–, CTLA4+, and Siglec-7– NK cells were increased in individuals with malaria experience (Figure 4A). Examining malaria-experienced individuals for common markers across all assays, 4-1BB+, CTLA-4+, CX3CR1+, NKG2C+, TIM-3+, and Siglec-7– NK cells were enriched for IL-10 production (Figure 4, B–D). This indicates that NK cells expressing these checkpoint (e.g., CTLA-4+, 4-1BB+, TIM-3+, Siglec-7–), adaptive (e.g., NKG2C+, Siglec-7–), and trafficking (CX3CR1+) markers are more likely to secrete IL-10. For the ADCC assay, additional markers, including CD16a+, KLRG1+, PD-1+, and TIGIT+, on NK cells were significantly enriched for IL-10 production (Figure 4C). Similar trends were seen in malaria-naive individuals, although the frequency of NK cells expressing individual molecules did vary substantially (Supplemental Figure 4A). Other markers tested were not significantly enriched for IL-10 on malaria-experienced individuals (Supplemental Figure 4B). We did not uncover a sole marker that predicts IL-10 production by NK cells. However, when assessing the coexpression of LAG-3+, NKG2C+, Siglec-7–, and TIGIT+ NK cells by SPICE analysis (Figure 4E), we found that around 25% of IL-10–secreting NK cells expressed all 4 markers and greater than 50% of the IL-10–secreting cells expressed at least 3 of the 4.
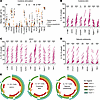 Figure 4
Figure 4IL-10 production from NK cell subsets for different functional assays for individuals with malaria experience. (A) Proportion of CD56+ NK cells expressing individual markers for malaria-naive (USA) and malaria-experienced (Mali) individuals after 6 days in IL-15 and IL-21 and stimulation with IL-12 (cytokine stimulation). (B–D) For individuals with malaria experience, proportion of IL-10 production from NK cell subsets for cytokine stimulation (B), ADCC (C), and natural cytotoxicity (D). (E) SPICE analysis for coexpression of selected markers for all 3 assays. Data were analyzed using Mann-Whitney U nonparametric tests with Bonferroni’s correction (14). *P < 0.05, **P < 0.01, ***P < 0.001 post Bonferroni correction.
NK cells produce IL-10 when cocultured with monocytes, Plasmodium-infected RBCs, and antibody. Coculture of NK cells with monocytes and infected RBCs can induce NK cells to produce IFN-γ (48). In the cytokine stimulation assays used in previous experiments, a key component to induce NK cells to produce maximal IL-10 was IL-12. IL-12 is secreted by monocytes and macrophages during NK cell and myeloid cell cross-talk (49). Monocytes make IL-12 in the process of engulfing infected RBCs (12). There are many inflammatory molecules in Plasmodium-infected RBCs, such as hemozoin, which activates the inflammasome pathway to induce IL-12 and IL-18 (50). Therefore, we hypothesized that, during coculture of NK cells with infected RBCs and monocytes, cytokines such as IL-12 made by monocytes would stimulate NK cells to produce IL-10. Furthermore, we hypothesized that the addition of anti-RBC antibodies would induce ADCC and potentially further increase IL-10 production. To test this, we used the coculture conditions laid out in Figure 5. We predict that infected and opsonized RBCs would cause inflammation through hemozoin and an ADCC/antibody-dependent cellular phagocytosis (ADCP) signal. For the ADCC/ADCP signals, 3 conditions were used: anti-RBC antibody that binds to both RBCs and infected RBCs, malaria-experienced immune plasma that will bind only infected RBCs, and malaria-naive plasma that should bind neither RBCs nor infected RBCs. NK cells from malaria-naive individuals incubated in IL-15 alone for 6 days were used as a negative control for IL-10 production. NK cells from malaria-naive individuals were also incubated with IL-15 and IL-21 for 6 days, where the proportion of IL-10 production is approximately 2% (Figure 5C). This control was used to make paired fold-change comparisons for CD107a, IFN-γ, and IL-10 production (Figure 5, A–C). Uninfected RBCs did not cause inflammation but could induce cytokine secretion from NK cells and monocytes if they were opsonized by an anti-RBC antibody causing ADCC and ADCP, respectively. We found that NK cells significantly increased degranulation (CD107a+) when there was an ADCC signal, and NK cells did not degranulate above baseline with infected-RBCs alone (Figure 5A). This is consistent with past evidence showing no significant natural cytotoxicity against infected RBCs (6–8). In agreement with previous studies, we found that NK cells, when cocultured with monocytes and infected RBCs, made significantly more IFN-γ (Figure 5B) (48, 51). We also found the NK cells made significant amounts of both IFN-γ and IL-10 with the addition of an ADCC/ADCP signal (Figure 5, B and C). The magnitude of the IL-10 production was largest in the group that included monocyte, infected RBCs, and an ADCC/ADCP signal (Figure 5C). This group also had the largest proportion of NK cells that were coexpressing IFN-γ and IL-10 (Figure 5D). We conclude that monocytes aide in inducing IL-10 secretion by NK cells.
 Figure 5
Figure 5Malaria-naive NK cell degranulation (CD107a), IFN-γ, and IL-10 production in an in vitro ADCC/ADCP assay with RBCs or P.falciparum–infected RBCs and purified malaria-naive monocytes. Column 1 is the condition with IL-15 alone. Column 2 is with IL-15 and IL-21. Subsequent conditions have the components on the left column added, indicated by a + symbol. (A–C) Degranulation (CD107a+) (A), IFN-γ+ (B), and IL-10+ (C) proportion between all the groups (left). The fold change (FC) of CD107a+ proportion relative to the IL-15 + IL-21 for the 6-day group (right). Data were analyzed via 1-way ANOVA with Tukey’s multiple-comparison test done comparing all groups to control IL-15 alone only group. (D) Comparison across groups of Boolean gates for IL-10 and IFN-γ. Red horizontal line is the median. **P < 0.01, ***P < 0.001, ****P < 0.0001.
-
Discussion
Clinical immunity to Plasmodium infection is achieved by controlling both parasite load and inflammation. One mechanism to clear infected RBCs and reduce parasitemia is cell-mediated clearance via Fc receptor recognition of antibodies bound to the surface of infected RBCs. This clearance can be through either phagocytosis by myeloid cells or by NK cell-directed ADCC (6–8, 12, 52–55). Our previous work demonstrated that adaptive NK cells correlate with reduced parasite load and reduced susceptibility to symptoms, indicating a potential mechanism of acquired trained immunity to malaria. Here, we provide potential insight into the contribution of NK cells regarding the control of inflammation through the production of the antiinflammatory cytokine IL-10.
We found that many more NK cells from individuals with malaria infection experience could secrete IL-10 as compared with NK cells from malaria-naive individuals. We hypothesize that this may be important for developing clinical immunity to malaria and preventing severe disease. Although the participants have undoubtedly been exposed to other infections aside from malaria, it stands to reason that an immune mechanism involved in the control of inflammation would be elevated in individuals experiencing repeated cycles of Plasmodium infection in the absence of severe disease. In support of this concept, we have also shown that IL-10 produced by NK cells in a mouse model of ECM is sufficient to protect from lethality (34). Additional studies examining NK cells from children who experience severe disease would be useful to determine if the IL-10–producing NK cell population is lacking in these susceptible individuals. Likewise, samples from individuals who experience high or low Plasmodium exposure would further strengthen the assertion that NK cells playing a regulatory role through production of IL-10 develop following repeated Plasmodium exposure (56). We hypothesize that increased IL-10 production is due to “peripheral training” of NK cells after repeated exposure to the inflammatory cytokine milieu of malaria (57); however, genetic elements may also contribute to an increased propensity to make IL-10. Previous studies have also shown that CD4+ T cells from individuals in malaria-endemic areas also display a marked increase in IL-10, which was dependent on malaria infection experience (30). Thus, there may be multiple types of immune cells engaged to limit inflammation while still allowing for the control of parasite load.
Additionally, we found that most IL-10–producing NK cells also make IFN-γ. Production of IFN-γ occurred rapidly ex vivo; however, NK cells only make IL-10 after being stimulated in vitro for 6 days (Supplemental Figure 3). We hypothesize this kinetic difference may be because the immune system first employs NK cells to stimulate the immune response to infection (through early IFN-γ) but then uses NK cells to diminish the response via IL-10 secretion and return to homeostasis, thereby preventing overt pathology. Similar “waves” of cytokine production by NK cells have been shown in mouse models of bacterial infection (47). Here, at the end of the 6-day culture period, we found that human NK cells were capable of concurrently producing IL-10 and IFN-γ. This was also observed in multiple publications that show that CD4+ T cells in malaria-experienced children were concurrently producing IL-10 and IFN-γ (30, 58–61). How these 2 seemingly contradictory signals would be perceived by responding immune cells is unclear and highlights the importance of understanding how complex cytokine networks affect the outcome of malaria or other infections.
We also found that NK cells could both degranulate (as demonstrated by CD107a+ expression) and secrete IL-10. NK cells have been shown to regulate the contraction phase of immune responses following viral infection through cytotoxic activity against immune cells (62–64). In conjunction with this activity, IL-10 may also dampen immune responses by inhibiting proliferation, antigen presentation, and the activity of cytokine signals. In contrast to this inhibitory activity, it has also been shown that IL-10 can increase the killing capacity of NK cells (65, 66). Others have shown that IL-10 induces metabolic changes in NK cells that enhance their cytotoxic functions (65, 66). From this, we postulated that NK cells secreting IL-10 may increase NK cell degranulation during ADCC and natural cytotoxicity.
Based on dual staining for degranulation and IL-10 production, we also found NK cells that were only degranulating (CD107a+) or only producing IL-10. We found that degranulating cells were enriched for TIGIT+ expression (IL-10+/–) while cells making IL-10 (CD107a+/–) were enriched in the Siglec-7– and CD45RO+ NK cell populations (Figure 3). These markers may identify 2 subsets of NK cells that have increased function and are also specialized for cytotoxicity or IL-10 production. Siglec-7 is an inhibitory sialic acid binding protein, so its loss would theoretically increase functions. TIGIT is also an inhibitory checkpoint molecule; therefore, its correlation with increased NK cell degranulation invokes a cell specific and context dependent role for TIGIT in NK cell regulation. There is precedent for checkpoint inhibitors inducing different outcomes when comparing NK cells and T cells. Tim-3+ T cells exhibit poor function, whereas Tim-3+ NK cells exhibit improved function (67, 68). Our data support this outcome in NK cells. How and why these checkpoint inhibitors have differing roles in individual cell types has yet to be determined.
Comparing NK cell IL-10 release in response to 3 different stimulations (cytokine stimulation, ADCC, and natural cytotoxicity) showed no difference between assays, although there was a trend toward a higher proportion of IL-10–secreting cells in the ADCC assay (Figures 1 and 2, and Supplemental Figure 1B). During ADCC, cytotoxic degranulation leads to the lysis of target cells with subsequent spilling of intracellular components that can promote inflammation (69). The lysis of infected RBCs has been shown to release molecules, such as heme and hemozoin, that can act as danger-associated molecular patterns (DAMPs), which activate multiple inflammatory pathways including TLR signaling, neutrophil extracellular trap release, and inflammasome formation (70, 71). Perhaps to dampen this effect, NK cells secrete the antiinflammatory cytokine IL-10 during or after cytotoxic degranulation. Mechanistic studies into the secretion of IL-10 during NK cell cytotoxic degranulation have not been reported.
The finding that IL-10 secretion is only stimulated by IL-15 in the presence of other cytokines, namely IL-21 and IL-12, is consistent with our previous study (34). IL-12 and IL-18, which are produced by myeloid cells, have effects on NK cell differentiation and can produce memory-like NK cells. Importantly, monocytes have also been shown to induce NK cells to produce IFN-γ in the presence of infected RBCs (48, 51). Our data support this finding but also show that NK cells can make IL-10 under this condition and have an even higher trending increase in cells producing IL-10 when stimulated to perform ADCC (Figure 5C). Our data indicate that either cell-cell contact or a secreted factor from monocytes induces IL-10 production by NK cells. We hypothesize that IL-12 is important for this effect based on our cytokine stimulation assay, which required the addition of IL-12 for maximal IL-10 production. However, further work will need to be done to determine whether cytokines (singly or in combination), cell-cell contact, or both are needed to induce IL-10 production by NK cells.
Lastly, IL-10 production by NK cells across all in vitro assays showed that 4-1BB+, CTLA–4+, Siglec-7–, NKG2C+, CX3CR1+, LAG-3+, and TIM-3+ NK cells had significant increases in IL-10 secretion (Figure 4, B and D). Additional markers that were significantly increased in the ADCC assay included TIGIT+, KLRG1+, and PD-1+ (Figure 4C). We found a wide range of phenotypic markers on IL-10–producing cells, suggesting that many NK cells can produce this cytokine under inflammatory conditions. These data indicate an enriched signature for a seemingly mixed array of adaptive and exhaustion markers. It also suggests that exhaustion markers are context dependent and may be beneficial for NK cells by promoting their regulatory functions under certain circumstances (e.g., during acute and chronic infections).
Greater IL-10 secretion from the NK cells of malaria-exposed individuals along with evidence of enhanced cytotoxicity and activation of these cells imply a dual role for NK cells as both important for parasite clearance and immunoregulation. NK cells may be key in mediating host immune responses to malaria infection to achieve the needed balance between a response strong enough to clear parasites but not so forceful as to cause immunopathology.
-
Methods
Sex as a biological variable. Both male and female participants were analyzed in this study, and similar findings are reported for both sexes.
Participants. Study participants from Kalifabougou, Mali, were enrolled in this study as part of an ongoing cohort study of acquired immunity to malaria (11). Characteristics of the donors whose samples were used in this manuscript are described in Supplemental Table 1. Blood was collected by venipuncture for up to 3 different time points for each study participant. The first blood collection was taken during May, before the malaria season began. If participants presented to the local health clinic with malaria symptoms, a second and third blood collection was obtained. The first was obtained during the malaria clinic visit, and the second as a convalescent time point 7 days later. Blood smears and rapid diagnostic tests (RDT) were performed for malaria parasitemia and diagnosis, respectively. Any participant with an RDT+ test was treated for malaria according to the National Malaria Control Program guidelines in Mali, which recommend artemether-lumefantrine for uncomplicated P. falciparum malaria. For this study, malaria was qualified as > 2,500 parasites per μL and a fever above 37.5°C. PBMCs were frozen as described previously (7). Briefly, after spinning BD Vacutainer CPT Mononuclear Cell Preparation Tube at manufacturer recommended speed (g) and time, plasma was aliquoted and frozen at –80°C. PBMCs were aspirated off the top of the gel, and then the gel was washed once more with RPMI+10%FBS. PBMCs were then counted. PBMCs were resuspended first in solution A (RPMI+50% FBS) in 0.5 mL and put in prelabeled cryogenic tubes. Once there were enough samples to freeze, solution B was added (FBS+15%DMSO). Then the tubes were quickly put in a prechilled (4°C) Mr. Frosty (Thermo Fisher Scientific) and put in the –80°C freezer for 24 hours. After 24 hours, they were moved to longer term storage in −196°C liquid nitrogen. Samples from Mali were shipped on dry ice (−78.5°C) by courier service to NIH, NIAID, Rockville, Maryland, USA, where they were again stored in liquid nitrogen. The samples were then forwarded, again on dry ice (−78.5°C) overnight using a courier service to University of Minnesota, Minneapolis, Minnesota, USA, where they were stored in liquid nitrogen before being used for experiments. Malaria-naive control samples were obtained from Memorial Blood Bank and PBMCs were processed and frozen as above, except percoll salt gradients (GE; Supplemental Table 2) were used to isolate PBMCs.
K562 cell maintenance. K562–artificial antigen-presenting cells (K562-aAPCs) were maintained between 1 × 105 to 1.5 × 106 cells/mL in RPMI-1640 (Thermo Fisher Scientific, SH3002701) + 10% FBS (PEAK Serum, PS-FB1) (RP10) + 1 μg/mL gentamicin (Sigma-Aldrich, G1272) and incubated at 37°C with 5% CO2. Unless listed otherwise, all 37°C incubations are conducted at 5% CO2.
Cytokine stimulation and cytotoxicity assays. PBMC vials were thawed, washed, and resuspended in 2 mL of culturing media (Lonza X-VIVO-15, serum-free hematopoietic cell medium, with L-glutamine, gentamicin, and phenol red, and 20% heat inactivated human AB serum; Peak Serum). Culture media was then added to each sample to create a concentration of 800,000 cells per mL. For each sample, 1 mL of this cell suspension was plated for day 0 experiments. Cells were rested overnight at 37°C and 5% CO2 before proceeding with cytotoxicity assays and cell staining as described below. To the remaining cell suspension, IL-15 (National Cancer Institute) was added to a final concentration of 20 ng/mL. This cell suspension was thoroughly mixed and plated into a 24-well plate to serve as the control day 6 sample. To the remaining cell suspension, recombinant human IL-21 (BioLegend) was added to a final concentration of 50 ng/mL. This cell suspension was then plated at 800,000 cells per well and incubated at 37°C and 5% CO2 for 6 days.
For the day 0 functional assays, 500,000 cells were plated into 3 wells (1 for the natural cytotoxicity assay, 1 for ADCC, and 1 for a negative control) per PBMC sample in a 96-well plate. For the ADCC and natural cytotoxicity assays, RBCs (Memorial Blood Centers, St. Paul Minnesota, USA) and K562 cells were resuspended to a concentration of 500,000 cells per 100 μL. RBCs were purified from whole blood by leukocyte reduction filtration (Fenwal Inc.) and resuspended at 50% hematocrit in RPMI-1640 and 25 mM HEPES, L-glutamine, and 50 mg/L Hypoxanthine (K-D Medical). For the natural cytotoxicity assay, K562 cells were added to sample PBMCs at a ratio of 1:1. For the ADCC assays, the RBC suspension was incubated with rabbit anti–human RBC antibodies (Supplemental Table 2) for 20 minutes (Rockland) before being added to 500,000 PBMCs at a 1:1 ratio in the 96-well plate, and the anti-RBC antibody was maintained in the culture for the duration of the assay (6, 7, 72–74). A 1:1,000 dilution of the anti-human RBC antibody was chosen because this caused minimal agglutination (<10%) while yielding a strong NK cell ADCC response. We chose RBCs as targets because they are the cell type infected by Plasmodium and they have very low background natural cytotoxicity (6, 7, 72–74). For the negative control, 100 μL of culturing media was added. All combinations were incubated at 37°C and 5% CO2 for 4 hours. Following the incubation period, a master mix of anti–human CD107a (BioLegend) and IL-10 catch reagent (Miltenyi Biotec) (Supplemental Table 2) were added per manufacturer’s instruction, and cells were incubated for 1 more hour at 37°C in 5% CO2.
Day 6 functional assays followed the protocol above with the following exceptions. On day 6, IL-12 was added to all wells (3 ng/mL) except those serving as the control (IL-15 only). The cytokine stimulation assay includes PBMCs stimulated with IL-15 (20 ng/mL), IL-21 (50 ng/mL) for 6 days, and IL-12 (3 ng/mL) for 5 hours. The ADCC and natural cytotoxicity assays were performed as on day 0 with the exception that IL-12 (3 ng/mL) was included (Figure 2). Cytokine concentrations were the same for the functional assays as for the cytokine stimulation assay. Quality control of staining was monitored using the same internal control (PBMCs derived from a single donor) in every batch of samples analyzed. For experiments where both IL-10 and IFN-γ were stained in the same sample, we found that adding IL-12 to the sample induced most of the NK cells to produce IFN-γ (data not shown). Therefore, when we did ADCC or natural cytotoxicity assays and assessed IFN-γ, we did not add IL-12 (Figure 3). We added anti-CD107a and IL-10 catch reagent into the wells at the beginning of the ADCC and natural cytotoxicity functional tests. Then after 3 hours, we added brefeldin A and monensin (MilliporeSigma) (10 μL total volume) (1 μg/mL Brefeldin A and monensin final). This allows the IL-10 to be released from the NK cells and detected on the outside of the cell, and then later for IFN-γ to be sequestered for detection by internal antibody staining.
Coculture NK ADCC and monocyte ADCP assay. Purified NK cells (STEMCELL human NK cell kit) were thawed and plated in a 24-well plate at a density of 800,000 cells/well in 1 mL Lonza media with IL-15 and IL-21 and incubated in a 37°C and 5%CO2 incubator for 6 days. On day 6, NK cells were resuspended at 100,000 cells/well and transferred to a 96-well plate. Monocytes were previously purified from whole blood (STEMCELL Technologies) and frozen similarly to PBMCs above. Monocytes were added at 100,000 cells/well, and RBCs and Plasmodium-infected RBCs (iRBCs; strain 3D7) were added at 200,000 cells/well. Mali plasma and US plasma were added at a dilution of 1:10 final concentration. The plate was then incubated for 5 hours. The plate was spun down at 550g for 4 minutes, and 115 μL of supernatant was carefully removed. In total, 15 μL of IL-10 catch reagent (Miltenyi Biotec) and anti-CD107a (1:200 final) mix were added to each well and incubated for another 2 hours. Then, after 2 more hours, 10 μL of Monensin/BFA solution (1 μg/mL final) was added to each well and incubated for 2 more hours. The cells were then centrifuged (500g), washed, and stained for flow cytometry.
Flow cytometry. Cells were resuspended in a master mix composed of PBS and viability dye (Tonbo) and incubated at 4°C for 30 minutes. At the end of the 30-minute incubation, cells were washed with FACS buffer (PBS, 2% FBS, 2 mM EDTA), stained with surface antibodies, and incubated at room temperature in the dark for 20 minutes. For assays with no internal stain, cells were incubated with 2% formaldehyde for 10 minutes before being washed and resuspended in PBS for flow cytometry analysis. If internal staining was performed, the cells were washed and incubated in 2% formaldehyde (Thermo Fisher Scientific) at 37°C for 10 minutes. After incubation, the cells were washed and resuspended in 0.04% Triton X-100 (Thermo Fisher Scientific) at room temperature in the dark for exactly 7 minutes. The cells were then washed and stained with an internal master mix in FACS buffer + 2% BSA. Cells were then incubated at room temperature in the dark for 120 minutes. After incubation, the cells were washed once with FACS buffer and resuspended for flow cytometry. Samples were run on a BD Fortessa flow cytometer according to the manufacturer’s instructions and analyzed using FlowJo 10.8.1. SPICE analysis was done using a combination of the Pestle program (version 2.0) and SPICE program (Version 6.1).
Single-cell RNA-Seq analysis. Single-cell RNA-Seq analysis was carried out using the ddSEQTM Single-Cell Isolator (Bio-Rad) and the SureCellTM WTA 3′ Library Prep Kit (Illumina). Magnetically enriched NK cells from participants naive to malaria were treated with cytokines IL-15, IL-21, IL-12, or IL-15 alone prior to capture on the ddSeq Single-Cell Isolator. Library preparation was done using SureCellTM WTA 3′ Library Prep Kit. Resulting libraries were analyzed for an appropriate length distribution using an Agilent TapeStation and were initially sequenced on the Illumina MiSeq Nano with read lengths: read 1 = 70 bp, index = 8 bp, and R2 = 76 bp. Deeper sequencing was then achieved using an Illumina NextSeq 550 High-ouput 2 × 75 flow cell (total of 6 samples). Per-cell gene expression was quantified for each sample using the BaseSpace SureCell RNA Single-Cell Analysis Workflow v1.2.0 from Illumina, which uses Isas v1.2.6-000184develop for analysis, STAR v2.5.2b for read alignment to the Homo sapiens UCSC hg38 genome, and SAMtools v 1.3. Data were processed downstream using the Seurat single-cell analysis pipeline v4 in R v 4.1.0 (75). Samples were filtered of low-quality (number gene Features < 200) cells before normalization with SCTransform (76) using 3,000 variable genes, and were filtered for batch correction using the Seurat integration procedure using the top 30 principal components (PCs) (77). UMAP visualizations and nearest neighbor clustering (Louvain algorithm) were performed using the top 6 PCs. Purified NK cells were used and single cells that were determined CD3– by gene expression were 0 and were labeled as IL-10+ if IL-10 raw counts ≥ 1. NK cells that were CD3– and IL10– were compared with NK cells that were NK cells that were CD3– and IL-10+ (baseline group) with the Wilcoxon rank-sum test using their SCT-normalized gene counts (assay=“SCT”, min.pct=0.1, logfc.threshold=0, only.pos=FALSE). Genes were considered differentially expressed if FDR < 0.05. Those DEGs were plotted using the Seurat DotPlot() function.
350 surface protein analysis (LEGENDScreen). To assess the expression of approximately 350 surface proteins at once on NK cells that produced IL-10, cells were analyzed using BioLegend’s LEGENDScreen Human PE Kit (BioLegend, 700007). Seven participants were incubated with IL-15 and IL-21 (as above) for 6 days. On the sixth day, IL-12 was added to promote NK cells to produce IL-10. The 7 participants were then barcoded with combinations of CD45 antibodies conjugated to 3 fluorophores, allowing us to test the 7 different participants in 1 well (A, B, C, AB, AC, BC, ABC). After barcoding, the samples were stained for CD56, CD3, CD14, Live/Dead, and IL-10, as in the above protocols. These cells were then pooled together and aliquoted into the BioLegend LEGENDScreen plates to be stained with an individual PE antibody in each well. The cells were then acquired on a CytoFLEX flow cytometer (Beckman Coulter) and analyzed via FlowJo 10.8.1.
Of the 350 antibodies in the LEGENDScreen, geometric mean fluorescence intensity (gMFI) expression and percentage of PE (%PE) of 134 protein markers were productively stained on the NK cells. Two-tailed, paired t tests were performed to determine significant differences between IL-10+ and IL-10– groups. The 134 protein P values were adjusted in R using the Benjamini-Hochberg procedure with the p.adjust() function in R. Proteins were deemed significant if the adjusted P < 0.05. The gMFI and %PE values for significant proteins were placed into separate dataframes in R. gMFI values were mean centered and scaled using the scale() function. The gMFI data were reshaped (reshape R package) using melt() to reformat for ggplot2 visualization before %PE values were appended. Final dataframe was plotted using ggplot() as a dotplot. The dot color represents the protein expression (gMFI), and the dot size represents the percent protein expression (%PE) for the significant proteins.
Statistics. Statistical significance is indicated by P values in figure legends and was determined by using Prism Versions 9 and 10 (GraphPad). The number of samples (n) and statistical comparison groups are indicated in figures and legends. Data were analyzed using 1-way ANOVA with Tukey’s multiple-comparison test, Mann-Whitney U nonparametric tests with Bonferroni’s correction, or Kruskal-Wallis test with Dunn’s post hoc comparisons as appropriate.
Study approval. The Ethics Committee of the Faculty of Medicine, Pharmacy, and Dentistry at the University of Sciences, Technique, and Technology of Bamako and the IRB of the National Institute of Allergy and Infectious Diseases, NIH, approved the Mali study. Written informed consent was obtained from adult participants and from the parents/guardians of participating children. The Mali study is registered at ClinicalTrials.gov (NCT01322581). The institutional review board of the University of Minnesota reviewed and approved this study.
Data availability. Single-cell RNA-Seq data sets have been deposited in the Gene Expression Omnibus (GEO; accession no. GSE266970). Supporting Data Values are included, containing all data points shown in the graphs and the values behind reported means and medians, with separate tabs for each figure panel or table.
-
Author contributions
SEH and GTH designed research studies. SAM, JKD, MAHC, JS, and MP conducted experiments. SAM, JKD, MAHC, JS, MP, and KSB acquired data. SAM, MM, JKD, MP, and GTH analyzed data. PDC and KS provided samples. SAM, SEH, and GTH wrote the manuscript. SAM, JKD, MAHC, MP, KSB, MM, PDC, KS, SEH, and GTH edited the manuscript. SAM and JKD are co–first authors; the order reflects the relative contribution to the original manuscript.
-
Acknowledgments
We gratefully acknowledge the following support services at the University of Minnesota that contributed to the research results reported: University Flow Cytometry Resource, University of Minnesota Genomics Center, and the Minnesota Supercomputing Institute. This work was supported by the following funding sources: NIH/NIAID R01AI143828 (to SEH), NIH/NIAID R01AI146031 (to GTH), and NIH/NIAID T32AI007313 (to JKD). The Mali study was supported by the Division of Intramural Research, NIAID/NIH.
Address correspondence to: Sara E. Hamilton, 2101 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.4928; Email: hamil062@umn.edu. Or to: Geoffrey T. Hart, 2231 6th St. SE, Minneapolis, Minnesota, 55455, USA. Phone: 612.626.3093; Email: hart0792@umn.edu.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2025, McNitt et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2025;10(9):e183076.https://doi.org/10.1172/jci.insight.183076.
-
References
- Venkatesan P. The 2023 WHO World malaria report. Lancet Microbe. 2024;5(3):e214.
- Crompton PD, et al. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol. 2014;32:157–187.
- Laurens MB. RTS,S/AS01 vaccine (Mosquirix™): an overview. Hum Vaccin Immunother. 2020;16(3):480–489.
- Mandala WL, et al. The role of different components of the immune system against P.falciparum malaria: Possible contribution towards malaria vaccine development. Mol Biochem Parasitol. 2021;246:111425.
- Kayentao K, et al. Subcutaneous administration of a monoclonal antibody to prevent malaria. N Engl J Med. 2024;390(17):1549–1559.
- Arora G, et al. NK cells inhibit P.falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. Elife. 2018;7:e36806.
- Hart GT, et al. Adaptive NK cells in people exposed to P.falciparum correlate with protection from malaria. J Exp Med. 2019;216(6):1280–1290.
- Ty M, et al. Malaria-driven expansion of adaptive-like functional CD56-negative NK cells correlates with clinical immunity to malaria. Sci Transl Med. 2023;15(680):eadd9012.
- Liao C, et al. Monocyte-derived macrophages expand the murine stress erythropoietic niche during the recovery from anemia. Blood. 2018;132(24):2580–2593.
- Kremsner PG, et al. Prognostic value of circulating pigmented cells in African children with malaria. J Infect Dis. 2009;199(1):142–150.
- Tran TM, et al. A molecular signature in blood reveals a role for p53 in regulating malaria-induced inflammation. Immunity. 2019;51(4):750–765.
- Xuan KM, et al. The role of monocytes in malaria infection. Cent Eur J Immunol. 2023;48(1):54–62.
- Haroun-Izquierdo A, et al. Adaptive single-KIR+NKG2C+ NK cells expanded from select superdonors show potent missing-self reactivity and efficiently control HLA-mismatched acute myeloid leukemia. J Immunother Cancer. 2022;10(11):e005577.
- Schlums H, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456.
- Holmes TD, et al. The transcription factor Bcl11b promotes both canonical and adaptive NK cell differentiation. Sci Immunol. 2021;6(57):eabc9801.
- Foley B, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–2674.
- Lopez-Verges S, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108(36):14725–14732.
- Zhang T, et al. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J Immunol. 2013;190(4):1402–1406.
- Tran TM, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to P.falciparum infection. Clin Infect Dis. 2013;57(1):40–47.
- Hendriksen IC, et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis. 2013;207(2):351–361.
- Milner DA, JrMalaria pathogenesis. Cold Spring Harb Perspect Med. 2018;8(1):a025569.
- Riggle BA, et al. Desperately seeking therapies for cerebral malaria. J Immunol. 2020;204(2):327–334.
- King T, Lamb T. Interferon-γ: the Jekyll and Hyde of malaria. PLoS Pathog. 2015;11(10):e1005118.
- Koppelman B, et al. Interleukin-10 down-regulates MAHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7(6):861–871.
- Go NF, et al. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990;172(6):1625–1631.
- Saxena A, et al. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74(1):27–34.
- Bravo M, et al. IL-15 complex-induced IL-10 enhances plasmodium-specific CD4+ T follicular helper differentiation and antibody production. J Immunol. 2024;212(6):992–1001.
- Kurup SP, et al. Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nat Med. 2017;23(10):1220–1225.
- Surette FA, et al. Extrafollicular CD4 T cell-derived IL-10 functions rapidly and transiently to support anti-Plasmodium humoral immunity. PLoS Pathog. 2021;17(2):e1009288.
- Portugal S, et al. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog. 2014;10(4):e1004079.
- O’Garra A, et al. IL-10–producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114(10):1372–1378.
- Ng TH, et al. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129.
- Othoro C, et al. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis. 1999;179(1):279–282.
- Burrack KS, et al. Interleukin-15 complex treatment protects mice from cerebral malaria by inducing interleukin-10-producing natural killer cells. Immunity. 2018;48(4):760–772.
- Khan M, et al. NK Cell-based immune checkpoint inhibition. Front Immunol. 2020;11:167.
- Chauhan SKS, et al. Harnessing NK cell checkpoint-modulating immunotherapies. Cancers (Basel). 2020;12(7):1807.
- Cao Y, et al. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct Target Ther. 2020;5(1):250.
- Li Z, et al. 4-1BB antibody enhances cytotoxic activity of natural killer cells against prostate cancer cells via NKG2D agonist combined with IL-27. Immunotherapy. 2022;14(13):1043–1053.
- Chester C, et al. 4-1BB agonism: adding the accelerator to cancer immunotherapy. Cancer Immunol Immunother. 2016;65(10):1243–1248.
- Sanseviero E, et al. Anti-CTLA-4 activates intratumoral NK cells and combined with IL15/IL15Rα complexes enhances tumor control. Cancer Immunol Res. 2019;7(8):1371–1380.
- Krzywinska E, et al. CD45 isoform profile identifies Natural Killer (NK) subsets with differential activity. PLoS One. 2016;11(4):e0150434.
- Fu X, et al. Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO- natural killer cells following stimulation with interleukin-12. Immunology. 2011;134(1):41–49.
- Hammer Q, et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. 2018;19(5):453–463.
- Ahmad F, et al. High frequencies of polyfunctional CD8+ NK cells in chronic HIV-1 infection are associated with slower disease progression. J Virol. 2014;88(21):12397–12408.
- Obeng-Adjei N, et al. Malaria-induced interferon-γ drives the expansion of Tbethi atypical memory B cells. PLoS Pathog. 2017;13(9):e1006576.
- Villegas-Mendez A, et al. Parasite-specific CD4+ IFN-γ+ IL-10+ T cells distribute within both lymphoid and nonlymphoid compartments and are controlled systemically by interleukin-27 and ICOS during blood-stage malaria infection. Infect Immun. 2016;84(1):34–46.
- Clark SE, et al. Bacterial manipulation of NK cell regulatory activity increases susceptibility to listeria monocytogenes infection. PLoS Pathog. 2016;12(6):e1005708.
- Newman KC, et al. Cross-talk with myeloid accessory cells regulates human natural killer cell interferon-gamma responses to malaria. PLoS Pathog. 2006;2(12):e118.
- Michel T, et al. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front Immunol. 2012;3:403.
- Pack AD, et al. Hemozoin-mediated inflammasome activation limits long-lived anti-malarial immunity. Cell Rep. 2021;36(8):109586.
- Baratin M, et al. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to P.falciparum. Proc Natl Acad Sci U S A. 2005;102(41):14747–14752.
- Cherif MK, et al. Is Fc gamma receptor IIA (FcγRIIA) polymorphism associated with clinical malaria and P.falciparum specific antibody levels in children from Burkina Faso? Acta Trop. 2015;142:41–46.
- Shi YP, et al. Fcgamma receptor IIa (CD32) polymorphism is associated with protection of infants against high-density P.falciparum infection. VII. Asembo Bay Cohort Project. J Infect Dis. 2001;184(1):107–111.
- Munde EO, et al. Association between Fcγ receptor IIA, IIIA and IIIB genetic polymorphisms and susceptibility to severe malaria anemia in children in western Kenya. BMC Infect Dis. 2017;17(1):289.
- Milner DA, JrThe systemic pathology of cerebral malaria in African children. Front Cell Infect Microbiol. 2014;4:104.
- Bowman NM, et al. Longevity of genotype-specific immune responses to P.falciparum merozoite surface protein 1 in Kenyan children from regions of different malaria transmission intensity. Am J Trop Med Hyg. 2016;95(3):580–587.
- Netea MG, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388.
- Dooley NL, et al. Single cell transcriptomics shows that malaria promotes unique regulatory responses across multiple immune cell subsets. Nat Commun. 2023;14(1):7387.
- Edwards CL, et al. IL-10–producing Th1 cells possess a distinct molecular signature in malaria. J Clin Invest. 2023;133(1):e153733.
- Jagannathan P, et al. Effective antimalarial chemoprevention in childhood enhances the quality of CD4+ T cells and limits their production of immunoregulatory interleukin 10. J Infect Dis. 2016;214(2):329–338.
- Boyle MJ, et al. Effector phenotype of P.falciparum–specific CD4+ T cells is influenced by both age and transmission intensity in naturally exposed populations. J Infect Dis. 2015;212(3):416–425.
- Waggoner SN, et al. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481(7381):394–398.
- Gyurova IE, et al. Natural killer cell regulation of B cell responses in the context of viral infection. Viral Immunol. 2020;33(4):334–341.
- Cox A, et al. Targeting natural killer cells to enhance vaccine responses. Trends Pharmacol Sci. 2021;42(9):789–801.
- Cai G, et al. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur J Immunol. 1999;29(9):2658–2665.
- Wang Z, et al. IL-10 Enhances human natural killer cell effector functions via metabolic reprogramming regulated by mTORC1 signaling. Front Immunol. 2021;12:619195.
- Gleason MK, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–3072.
- Dixon KO, et al. Beyond T cell exhaustion: TIM-3 regulation of myeloid cells. Sci Immunol. 2024;9(93):eadf2223.
- Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126.
- Ferreira A, et al. A central role for free heme in the pathogenesis of severe malaria: the missing link? J Mol Med (Berl). 2008;86(10):1097–1111.
- Mendonca R, et al. Red cell DAMPs and inflammation. Inflamm Res. 2016;65(9):665–678.
- Dahlvang JD, et al. Ablation of SYK kinase from expanded primary human NK Cells via CRISPR/Cas9 enhances cytotoxicity and cytokine production. J Immunol. 2023;210(8):1108–1122.
- Dick JK, et al. Antibody-mediated cellular responses are dysregulated in Multisystem Inflammatory Syndrome in Children (MIS-C). J Pediatric Infect Dis Soc. 2024;13(4):8–9.
- Dick JK, Hart GT. Natural killer cell Antibody-Dependent Cellular Cytotoxicity (ADCC) activity against P.falciparum–infected red blood cells. Methods Mol Biol. 2022;2470:641–657.
- Butler A, et al. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–420.
- Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20(1):296.
- Stuart T, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–1902.
-
Version history
- Version 1 (May 8, 2025): Electronic publication








