Metabolism
Abstract
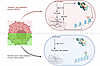
Dysfunctional white adipose tissue contributes to the development of obesity-related morbidities, including insulin resistance, dyslipidemia, and other metabolic disorders. Adipose tissue macrophages (ATMs) accumulate in obesity and play both beneficial and harmful roles in the maintenance of adipose tissue homeostasis and function. Despite their importance, the molecules and mechanisms that regulate these diverse functions are not well understood. Lipid-associated macrophages (LAMs), the dominant subset of obesity-associated ATMs, accumulate in crown-like structures and are characterized by a metabolically activated and proinflammatory phenotype. We previously identified CD9 as a surface marker of LAMs. However, the contribution of CD9 to the activation and function of LAMs during obesity is unknown. Using a myeloid-specific CD9 knockout model, we show that CD9 supports ATM-adipocyte adhesion and crown-like structure formation. Furthermore, CD9 promotes the expression of pro-fibrotic and extracellular matrix remodeling genes. Loss of myeloid CD9 reduces adipose tissue fibrosis, increases visceral adipose tissue accumulation, and improves global metabolic outcomes during diet-induced obesity. These results identify CD9 as a causal regulator of pathogenic LAM functions, highlighting CD9 as a potential therapeutic target for treating obesity-associated metabolic disease.
Authors
Julia Chini, Nicole DeMarco, Dana V. Mitchell, Sam J. McCright, Kaitlyn M. Shen, Divyansi Pandey, Rachel L. Clement, Jessica Miller, Rajan Jain, Deanne M. Taylor, Mitchell A. Lazar, David A. Hill
Abstract
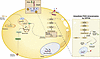
Nicotinamide adenine dinucleotide (NAD⁺) is essential for cellular metabolism, DNA repair, and stress responses. NAD+ is synthesized from nicotinamide, nicotinic acid (collectively termed niacin), and tryptophan. In humans, deficiencies in these nutrients result in pellagra, marked by dermatitis, diarrhea, and dementia. The dermatitis associated with pellagra typically manifests as photodermatosis in sun-exposed areas. This study examined the effects of NAD+ deficiency on skin homeostasis using epidermis-specific Nampt conditional knockout (cKO) mice. These mice displayed substantial NAD⁺ depletion, reduced poly(ADP-ribose) polymerase (PARP) activity, and increased DNA damage. Consequently, Nampt cKO mice developed spontaneous skin inflammation and epidermal hyperplasia. RNA sequencing and immunohistochemical analyses demonstrated increased interleukin-36 (IL-36) cytokine expression, suggesting that DNA repair-related genomic stress triggers keratinocyte-driven IL-36 production, which promotes inflammation. Furthermore, reduced collagen17A1 expression and elevated thymic stromal lymphopoietin (TSLP) levels were observed. NAD+ repletion by transdermal supplementation of nicotinamide mononucleotide (NMN) suppressed the rise of IL-36 levels and skin inflammation. These findings underscore the importance of Nampt-mediated NAD⁺ metabolism for epidermal stability and indicate that NAD⁺ depletion may contribute to IL-36-mediated skin inflammation, offering insights for therapeutic strategies in inflammatory skin disorders.
Authors
Taiki Seki, Jun-Dal Kim, Yasuhito Yahara, Hitoshi Uchida, Keisuke Yaku, Mariam Karim, Teruhiko Makino, Tadamichi Shimizu, Takashi Nakagawa
Abstract
Mitochondria-derived acyl-coenzyme A (acyl-CoA) species chemically modify proteins, causing damage when acylation reactions are not adequately detoxified by enzymatic removal or protein turnover. Defects in genes encoding the mitochondrial respiratory complex and TCA cycle enzymes have been shown to increase acyl-CoA levels due to reduced enzymatic flux and result in proteome-wide hyperacylation. How pathologically elevated acyl-CoA levels contribute to bioenergetics failure in mitochondrial diseases is not well understood. Here, we demonstrate that bulk succinylation from succinyl-CoA excess consumes the enzymatic cofactor NAD+ and propagates mitochondrial respiratory defects in a zebrafish model of succinyl-CoA ligase deficiency, a childhood-onset encephalomyopathy. To explore this mechanism as a therapeutic target, we developed a workflow to monitor behavioral defects in sucla2–/– zebrafish and show that hypersuccinylation is associated with reduced locomotor behavior and impaired ability to execute food hunting patterns. Postembryonic NAD+ precursor supplementation restores NAD+ levels and improves locomotion and survival of sucla2–/– zebrafish. Mechanistically, nicotinamide and nicotinamide riboside require the NAD+-dependent desuccinylase Sirt5 to enhance oxidative metabolism and nitrogen elimination through the urea cycle. Collectively, NAD+ supplementation activates Sirt5 to protect against damage to mitochondria and locomotor circuits caused by protein succinylation.
Authors
Joy Richard, Giulia Lizzo, Noélie Rochat, Adrien Jouary, Pedro T.M. Silva, Alice Parisi, Stefan Christen, Sofia Moco, Michael B. Orger, Philipp Gut
Abstract
Neutrophils play a pivotal role in the progression of metabolic dysfunction–associated steatohepatitis (MASH) by mediating inflammatory responses. However, the heterogeneity of neutrophil subsets in MASH and their specific contributions to disease progression remain unclear. In this study, analysis of liver biopsies from 265 patients revealed a strong association between elevated neutrophil counts and MASH severity, particularly fibrosis. Five distinct neutrophil subsets were identified in human liver tissue, with PAD4+ neutrophils serving as key drivers in MASH progression. Mechanistically, PAD4+ neutrophils generate neutrophil extracellular traps (NETs) and activate hepatic stellate cells via the TAOK1-dependent MAPK signaling pathway. Inhibition of PAD4+ neutrophils in vivo attenuated the progression of liver fibrosis without exacerbating liver injury. Collectively, these findings elucidate the pivotal involvement of PAD4+ neutrophils in MASH progression and identify them as promising therapeutic targets for mitigating fibrosis and inflammation.
Authors
Jiajia Shen, Shanshan Huang, Yaohui Wang, Qingyuan Wang, Shibo Lin, Wei Guan, Yingyun Gong, Yiming Si, Ming Zhao, Hongwen Zhou, Hui Liang
Abstract
Iron regulatory protein 1 (IRP1) is a post-transcriptional regulator of cellular iron metabolism. In mice, loss of IRP1 causes polycythemia through translational de-repression of hypoxia-inducible factor 2α (HIF2α) mRNA, which increases renal erythropoietin production. Here we show that Irp1-/- mice develop fasting hypoglycemia and are protected against high-fat diet–induced hyperglycemia and hepatic steatosis. Discovery-based proteomics of Irp1-/- livers revealed a mitochondrial dysfunction signature. Seahorse flux analysis in primary hepatocytes and differentiated skeletal muscle myotubes confirmed impaired respiratory capacity, with a shift from oxidative phosphorylation to glycolytic ATP production. This metabolic rewiring was associated with enhanced insulin sensitivity and increased glucose uptake in skeletal muscle. Under metabolic stress, IRP1 deficiency altered the redox balance of mitochondrial iron, resulting in inefficient energy production and accumulation of amino acids and metabolites in skeletal muscle, rendering them unavailable for hepatic gluconeogenesis. These findings identify IRP1 as a critical regulator of systemic energy homeostasis.
Authors
Wen Gu, Nicole Wilkinson, Carine Fillebeen, Darren Blackburn, Korin Sahinyan, Eric Bonneil, Tao Zhao, Zhi Luo, Vahab Soleimani, Vincent Richard, Christoph H. Borchers, Albert Koulman, Benjamin Jenkins, Bernhard Michalke, Hans Zischka, Judith Sailer, Vivek Venkataramani, Othon Iliopoulos, Gary Sweeney, Kostas Pantopoulos
Abstract
Enhanced lipid metabolism, which involves the active import, storage, and utilization of fatty acids from the tumor microenvironment, plays a contributory role in malignant glioma transformation; thereby, serving as an important gain of function. In this work, through studies initially designed to understand and reconcile possible mechanisms underlying the anti-tumor activity of a high-fat ketogenic diet, we discovered that this phenotype of enhanced lipid metabolism observed in glioblastoma may also serve as a metabolic vulnerability to diet modification. Specifically, exogenous polyunsaturated fatty acids (PUFA) demonstrate the unique ability of short-circuiting lipid homeostasis in glioblastoma cells. This leads to lipolysis-mediated lipid droplet breakdown, an accumulation of intracellular free fatty acids, and lipid peroxidation-mediated cytotoxicity, which was potentiated when combined with radiation therapy. Leveraging this data, we formulated a PUFA-rich modified diet that does not require carbohydrate restriction, which would likely improve long-term adherence when compared to a ketogenic diet. The modified PUFA-rich diet demonstrated both anti-tumor activity and potent synergy when combined with radiation therapy in mouse glioblastoma models. Collectively, this work offers both a mechanistic understanding and a potentially translatable approach of targeting this metabolic phenotype in glioblastoma through diet modification and/or nutritional supplementation that may be readily integrated into clinical practice.
Authors
Shiva Kant, Yi Zhao, Pravin Kesarwani, Kumari Alka, Jacob F. Oyeniyi, Ghulam Mohammad, Nadia Ashrafi, Stewart F. Graham, C. Ryan Miller, Prakash Chinnaiyan
Abstract
Obesity and type 2 diabetes (T2D) are metabolic diseases with increasing prevalence worldwide. Obesity often leads to T2D. Insulin resistance and impaired β cell function contribute to the onset of hyperglycemia. Previously, we reported that ablation of Gc, encoding a secreted protein with a primary role in vitamin D transport, improved pancreatic β cell function in models of diet-induced insulin resistance. Here, we show that Gc ablation had systemic insulin-sensitizing effects to prevent weight gain, hyperglycemia, and glucose intolerance; lower nonesterified fatty acids and triglycerides; and augment glucose uptake in skeletal muscle and adipose in male mice fed a high-fat diet. Interestingly, weight loss in Gc-ablated mice resulted from selective fat mass loss with preserved lean mass. Moreover, acute Gc inhibition prevented glucose intolerance caused by high-fat feeding. The data suggest that Gc inhibition can increase insulin production in β cells and insulin action in peripheral tissues, while reducing fat mass.
Authors
Richard Gill, Taiyi Kuo
Abstract
Metabolic inflammation is closely linked to dynamic changes in circulating monocyte populations, yet how nutritional signals regulate this process remains unclear. ANGPTL8, a hepatokine rapidly induced by refeeding, emerged as a key regulator of postprandial monocyte dynamics. We examined ANGPTL8 expression in human and murine fasting-refeeding models and manipulated ANGPTL8 expression specifically in hepatocytes to assess its role in metabolic inflammation and insulin resistance in obese mice. ANGPTL8 overexpression elevated circulating monocytes and proinflammatory cytokines, while its deletion reduced these parameters and conferred metabolic benefits. Mechanistically, recombinant ANGPTL8 stimulated CCL5 production in bone marrow-derived macrophages via P38 signaling activation, promoting monocyte recruitment and proinflammatory macrophage polarization. These effects were mitigated by CCR5 antagonism. Rescue experiments demonstrated that CCL5 supplementation in Angptl8-deficient mice restored monocyte levels and inflammatory responses. Functionally, ANGPTL8 worsened insulin resistance and glucose intolerance in obese mice, effects that were reversed by its deletion and recapitulated by CCL5 administration. These findings suggest that ANGPTL8 functions as a nutritional checkpoint linking feeding status to monocyte-mediated inflammation through the CCL5-CCR5 axis. By driving monocytosis and proinflammatory macrophage activation, ANGPTL8 exacerbates metabolic dysfunction. Targeting the ANGPTL8-CCL5-CCR5 pathway may therefore offer a promising therapeutic strategy for managing obesity-related metabolic diseases.
Authors
Ran-Ran Kan, Si-Yi Wang, Xiao-Yu Meng, Li Huang, Yu-Xi Xiang, Bei-Bei Mao, Hua-Jie Zou, Ya-Ming Guo, Li-Meng Pan, Pei-Qiong Luo, Yan Yang, Zhe-Long Liu, De-Lin Ma, Wen-Jun Li, Yong Chen, Dan-Pei Li, Xue-Feng Yu
Abstract
Therapeutics blocking PI3K/mTOR complex 1 (mTORC1) are commonly used for tumor treatment, and at times achieve major responses, yet minimal residual disease (MRD) persists, leading to tumor relapse. We developed multiple MRD models both in vitro (rapamycin persistent, RP) and in vivo after mTORC1 inhibition. All 11 RP/MRD cell lines showed complete growth and signaling insensitivity to rapamycin but variable sensitivity to bi-steric mTORC1 inhibitors, with MtorS2035 mutations identified in 4 of 7 RP cell lines. Multiomic analyses identified a pronounced shift toward oxidative phosphorylation and away from glycolysis with increased mitochondrial number in all RP/MRD models. MYC and SWI/SNF expression was significantly enhanced. Both the SWI/SNF inhibitor AU-15330 and the mitochondrial complex I oxidative phosphorylation inhibitor IACS-010759 showed pronounced synergy with bi-steric mTORC1 inhibitors to cause cuproptotic cell death in RP/MRD cells, suggesting these combinations as a potential patient treatment strategy for rapalog resistance.
Authors
Heng Du, Heng-Jia Liu, Magdalena Losko, Yu Chi Yang, Min Yuan, Elizabeth P. Henske, John M. Asara, Mallika Singh, David J. Kwiatkowski
Abstract
Processes that promote white adipocyte inflammatory function remain incompletely defined. Here, we demonstrated that type I interferon–dependent (IFN-I–dependent) skewing of adipocyte glycolysis, nicotinamide adenine dinucleotide (NAD+) utilization, and pyruvate kinase isozyme M2 (PKM2) function may contribute to increased systemic and tissue inflammation and disease severity in obesity. Notably, chemical and/or genetic inhibition of glycolysis, the NAD+ salvage pathway, or PKM2 restricted IFN-I–dependent increase in adipocyte inflammatory cytokine production. Further, genetic or small molecule targeting of PKM2 function in vivo was sufficient to reduce systemic and tissue inflammation and metabolic disease severity in obese mice, in an adipocyte PKM2-dependent manner. Further, white adipose tissue of individuals living with obesity and metabolic disease, compared with metabolically healthy individuals with obesity, showed an increase in expression of inflammatory and metabolic genes, while small molecule targeting of PKM2 function contributed to reduced IFN-I–driven inflammatory cytokine production by primary human adipocytes. Together, our findings invoke the IFN-I/PKM2 axis as a potential target for modulating adipocyte dysregulated inflammation.
Authors
Michelle S.M.A. Damen, Pablo C. Alarcon, Calvin C. Chan, Traci E. Stankiewicz, Hak Chung, Keisuke Sawada, Cassidy J. Ulanowicz, John Eom, Jarren R. Oates, Jennifer L. Wayland, Jessica R. Doll, Rajib Mukherjee, Miki Watanabe-Chailland, Lindsey Romick-Rosendale, Sara Szabo, Michael A. Helmrath, Joan Sanchez-Gurmaches, Maria E. Moreno-Fernandez, Senad Divanovic
No posts were found with this tag.