Research ArticleImmunologyInflammation
Open Access |  10.1172/jci.insight.194201
10.1172/jci.insight.194201
Delineating the short- and long-term impact of ionizing radiation on antigen-inexperienced CD8+ T cell subsets
Mohammad Heidarian,1,2 Shravan K. Kannan,1,3 Whitney Swanson,4 Thomas S. Griffith,4,5,6 John T. Harty,1,2,3 and Vladimir P. Badovinac1,2,3
1Department of Pathology,
2Experimental Pathology Graduate Program, and
3Interdisciplinary Graduate Program in Immunology, University of Iowa, Iowa City, Iowa, USA.
4Department of Urology and
5Center for Immunology, University of Minnesota, Minneapolis, Minnesota, USA.
6Minneapolis VA Health Care System, Minneapolis, Minnesota, USA.
Address correspondence to: Vladimir P. Badovinac, Department of Pathology, The University of Iowa, 1020 Medical Laboratories, 25 S. Grand Avenue, Iowa City, Iowa 52246, USA. Phone: 319.384.2930; Email: Vladimir-badovinac@uiowa.edu.
Find articles by Heidarian, M. in: PubMed | Google Scholar
1Department of Pathology,
2Experimental Pathology Graduate Program, and
3Interdisciplinary Graduate Program in Immunology, University of Iowa, Iowa City, Iowa, USA.
4Department of Urology and
5Center for Immunology, University of Minnesota, Minneapolis, Minnesota, USA.
6Minneapolis VA Health Care System, Minneapolis, Minnesota, USA.
Address correspondence to: Vladimir P. Badovinac, Department of Pathology, The University of Iowa, 1020 Medical Laboratories, 25 S. Grand Avenue, Iowa City, Iowa 52246, USA. Phone: 319.384.2930; Email: Vladimir-badovinac@uiowa.edu.
Find articles by Kannan, S. in: PubMed | Google Scholar
1Department of Pathology,
2Experimental Pathology Graduate Program, and
3Interdisciplinary Graduate Program in Immunology, University of Iowa, Iowa City, Iowa, USA.
4Department of Urology and
5Center for Immunology, University of Minnesota, Minneapolis, Minnesota, USA.
6Minneapolis VA Health Care System, Minneapolis, Minnesota, USA.
Address correspondence to: Vladimir P. Badovinac, Department of Pathology, The University of Iowa, 1020 Medical Laboratories, 25 S. Grand Avenue, Iowa City, Iowa 52246, USA. Phone: 319.384.2930; Email: Vladimir-badovinac@uiowa.edu.
Find articles by Swanson, W. in: PubMed | Google Scholar
1Department of Pathology,
2Experimental Pathology Graduate Program, and
3Interdisciplinary Graduate Program in Immunology, University of Iowa, Iowa City, Iowa, USA.
4Department of Urology and
5Center for Immunology, University of Minnesota, Minneapolis, Minnesota, USA.
6Minneapolis VA Health Care System, Minneapolis, Minnesota, USA.
Address correspondence to: Vladimir P. Badovinac, Department of Pathology, The University of Iowa, 1020 Medical Laboratories, 25 S. Grand Avenue, Iowa City, Iowa 52246, USA. Phone: 319.384.2930; Email: Vladimir-badovinac@uiowa.edu.
Find articles by
Griffith, T.
in:
PubMed
|
Google Scholar
|

1Department of Pathology,
2Experimental Pathology Graduate Program, and
3Interdisciplinary Graduate Program in Immunology, University of Iowa, Iowa City, Iowa, USA.
4Department of Urology and
5Center for Immunology, University of Minnesota, Minneapolis, Minnesota, USA.
6Minneapolis VA Health Care System, Minneapolis, Minnesota, USA.
Address correspondence to: Vladimir P. Badovinac, Department of Pathology, The University of Iowa, 1020 Medical Laboratories, 25 S. Grand Avenue, Iowa City, Iowa 52246, USA. Phone: 319.384.2930; Email: Vladimir-badovinac@uiowa.edu.
Find articles by
Harty, J.
in:
PubMed
|
Google Scholar
|

1Department of Pathology,
2Experimental Pathology Graduate Program, and
3Interdisciplinary Graduate Program in Immunology, University of Iowa, Iowa City, Iowa, USA.
4Department of Urology and
5Center for Immunology, University of Minnesota, Minneapolis, Minnesota, USA.
6Minneapolis VA Health Care System, Minneapolis, Minnesota, USA.
Address correspondence to: Vladimir P. Badovinac, Department of Pathology, The University of Iowa, 1020 Medical Laboratories, 25 S. Grand Avenue, Iowa City, Iowa 52246, USA. Phone: 319.384.2930; Email: Vladimir-badovinac@uiowa.edu.
Find articles by
Badovinac, V.
in:
PubMed
|
Google Scholar
|

Published August 5, 2025 - More info
JCI Insight. 2025;10(18):e194201. https://doi.org/10.1172/jci.insight.194201.
© 2025 Heidarian et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Received: April 4, 2025; Accepted: July 31, 2025
-
Abstract
Radiation-induced lymphopenia (RIL) remains a challenging side effect of radiation therapy that is often associated with poor prognosis and reduced overall survival. Although CD8+ T cells are highly radiosensitive, the dynamics of quantitative and qualitative changes to the CD8+ T cell pool following exposure to high doses of ionizing radiation (IR) remain understudied. Herein, we sought to determine the long-term impact of sublethal whole body irradiation (WBI) on the antigen-inexperienced (Ag-inexperienced) CD8+ T cell pool, comprising naive (TN) and virtual memory (TVM) CD8+ T cells. We show that although both TN and TVM cells gradually regenerated after WBI-induced loss, TN recovery occurred only through de novo thymic production. Despite the numerical restoration, the subset and phenotypic composition of postrecovery Ag-inexperienced CD8+ T cells did not qualitatively recapitulate the pre-WBI state. Specifically, the frequency of TVM cells is increased, especially during the early stages of recovery. Within the TN subset, a lasting overrepresentation of Ly6C+CD122+ cells and an altered TCR clonotype diversity are also observed. Overall, our data highlight the dynamic changes to the Ag-inexperienced CD8+ T cell pool upon recovery from RIL
-
Introduction
Radiotherapy is a common approach used in more than 50% of solid tumor treatment plans. Ionizing radiation (IR) can directly kill tumor cells through induction of DNA damage and act as an “in situ vaccine” to trigger antitumor immune response (1–3). IR can also cause deleterious side effects on healthy tissues (4–6). For example, lymphocytes are one of the most radiosensitive cells among the immune system. Although cells of lymphoid origin differ in their susceptibility to IR-induced cell death, with B cells being the most radiosensitive, followed by T and NK cells, even low doses of IR (as little as 0.5 Gy) can rapidly induce lymphocyte death (7–9). In fact, more than 40% of patients with solid tumors receiving radiotherapy as part of their treatment regimen develop severe lymphopenia (10), which is associated with poor prognosis and reduced overall survival (11, 12). In addition to increased susceptibility to both previously and newly encountered pathogens, prolonged radiation-induced lymphopenia (RIL) has been associated with decreased efficacy of immunotherapy approaches (13, 14). While treatment plans with minimal unwanted IR exposure to lymphocyte-rich niches have been proposed to prevent RIL (15, 16), further understanding of the factors that contribute to qualitative and quantitative recovery of lymphocytes paves the path to a more efficient treatment paradigm.
CD8+ T cells are potent mediators of immunity against intracellular pathogens and malignancies (17–19). At steady state, naive CD8+ T (TN) cells remain quiescent, but cognate antigen (Ag) encounter along with costimulatory and inflammatory signals trigger massive proliferation and differentiation of TN cells to activated effector CD8+ T cells that combat the infected/cancerous cells and eventually give rise to long-lasting bona fide memory CD8+ T (TMEM) cells (20–23). While continuous TCR contact with self-antigens and homeostatic cytokines are required for the long-term survival of TN cells (24–26), these signals can also mediate the transition of TN cells to the semidifferentiated virtual memory CD8+ T (TVM) cells that share phenotypic similarities with TMEM cells. TVM cells generally represent a smaller subset of Ag-inexperienced CD8+ T cells than TN cells (27–29). Maintaining a numerically stable and heterogeneous population of Ag-experienced and -inexperienced CD8+ T cells with a highly diverse repertoire of unique TCRs is crucial in conferring protection against a wide range of Ags (30–33).
Thymic production and homeostatic proliferation (HP) of peripheral T cells are 2 mechanisms that can regenerate the T cell compartment under lymphopenic conditions (34, 35). Owing to the higher capacity of CD8+ T cells to undergo HP than CD4+ T cells, numerical recovery of CD8+ T cells is less dependent on thymic output (36, 37). The capacity of CD8+ T cells to undergo HP also does not decline with age, unlike the thymic output, making HP the major regenerative mechanism for CD8+ T cells in adults (36, 38). Although HP may reestablish the number of CD8+ T cells, it can result in preferential recovery of certain TCR clones and result in altered clonal diversity of the CD8+ T cell compartment, highlighting the importance of de novo thymic emigrants to balance CD8+ T cell recovery (39, 40). Here, we investigated the kinetics of recovery and composition of the Ag-inexperienced CD8+ T cells following sublethal whole body irradiation (WBI). We also examined the contribution of newly generated T cells from thymus and HP of IR-experienced CD8+ T cells to the recovery of Ag-inexperienced CD8+ T cell pool. Our results suggest that unlike bona fide TMEM cells, Ag-inexperienced CD8+ T cells were gradually restored. In the absence of a thymus, TN recovery was severely compromised, and CD8+ T cells that survived the IR exposure only gave rise to TVM cells. Euthymic mice, on the other hand, were able to regenerate both subsets of Ag-inexperienced CD8+ T cells, with the postrecovery TN cells appearing to be phenotypically and clonally distinct. These data collectively suggest that exposure to high doses of IR can have a profound, long-term impact on the composition of Ag-inexperienced CD8+ T cells.
-
Results
Ag-inexperienced CD8+ T cells are rapidly lost but gradually restored following sublethal WBI. Bona fide TMEM cells undergo a permanent attrition following exposure to a 5 Gy dose of WBI (41). To compare the kinetic of loss and recovery of Ag-inexperienced CD8+ T cells and bona fide TMEM cells that are cognate Ag-experienced cells, we exposed lymphocytic choriomeningitis virus–experienced (LCMV-experienced) P14 chimeric mice to either mock or sublethal WBI (Figure 1A). To enumerate TN cells, we used a surrogate marker approach by which TN cells can be distinguished from CD8+ T cells with Ag-experienced/memory phenotype based on higher expression of CD8α and lower expression of CD11a, a component of integrin molecule lymphocyte function-associated antigen 1(42, 43). TN cells demonstrated a tissue-wide and dose-dependent depletion as early as 1 day after exposure to different doses of WBI (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.194201DS1). Consistent with the previous results (41), the number of TN cells, but not memory P14 cells, started to increase between day 10 (D10) and D20 after WBI and steadily rose to repopulate the TN compartment (Figure 1, B–D). We also investigated the numerical changes to TVM T cells in the same hosts following WBI. TVM cells are Ag-inexperienced CD8+ T cells that express many markers found on bona fide TMEM cells despite lacking prior Ag exposure (27). CD49d, an integrin upregulated only on effector and Ag-experienced memory CD8+ T cells, is a key marker used to distinguish TVM cells from true Ag-experienced and TMEM cells (44–46). Despite sharing the memory phenotype with bona fide TMEM cells, TVM cells followed a similar pattern of gradual numerical recovery as the TN cells. In fact, the TVM cells appeared to be overrepresented in irradiated hosts for extended periods after WBI (Figure 1E). These findings indicated that unlike bona fide TMEM cells, the Ag-inexperienced CD8+ T compartment, namely TN and TVM cells, numerically recover following WBI.
 Figure 1
Figure 1Numbers of Ag-inexperienced and memory CD8+ T cells are differentially altered following WBI. (A) Experimental design: 104 naive Thy1.1+ P14 CD8+ T cells were adoptively transferred into Thy1.2+ naive hosts, followed by LCMV-Armstrong infection to generate memory P14 CD8+ T cells. At 45 days later, the memory P14 chimeric mice were exposed to mock (0 Gy) or 5 Gy dose of WBI. Analysis was performed on the indicated time points after irradiation from peripheral blood lymphocytes (PBL). Dx, days after WBI. (B) Representative flow plots of naive (TN) and virtual memory (TVM) CD8+ T cells and memory P14 cells from D20 after WBI 0 Gy and 5 Gy mice. Numbers of (C) TN cells, (D) memory P14 cells, and (E top panel) TVM cells were assessed at indicated time points in PBL analysis. (E bottom panel) Frequency of TVM cells of total CD8+ T cells was also determined. Data in A–E are representative of 2 independent experiments with n = 5–10 mice per group in each experiment. Statistical significance was determined by 2-way ANOVA with Bonferroni’s multiple comparisons post hoc test using GraphPad Prism. Graphs show the mean ± SEM with each symbol representing 1 mouse. Individual P values are noted on respective graphs or are summarized as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
We next investigated the loss and recovery of Ag-inexperienced CD8+ T cells in specific pathogen–free (SPF) mice following WBI (Figure 2A). To define TN and TVM cells, we used a different gating strategy where TN (CD44lo) and TVM (CD44hiCD49dlo) were distinguished from true Ag-experienced (CD44hiCD49dhi) CD8+ T cells in SPF mice (Figure 2B). Following a WBI-mediated loss of TN cells, vigorous proliferation of TN cells ensued, as demonstrated by a substantial increase in the expression of Ki67 in TN cells, leading to a substantial numerical recovery of TN cells between D10 and D25 after WBI and ultimately returning to the baseline values by D60 (Figure 2C). TVM cells also demonstrated a similar pattern of loss and recovery as TN cells; however, the overrepresentation of TVM was only observed until the TN pool was fully reconstituted (Figure 2D). These data collectively suggest that TN and TVM cells, despite their phenotypic differences, follow a similar numerical drop and recovery following WBI in hosts with pathogen a history of pathogen exposure and SPF hosts.
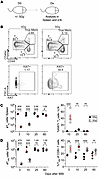 Figure 2
Figure 2Naive and virtual memory CD8+ T cells follow similar kinetic of loss and recovery following WBI. (A) Experimental design: specific pathogen–free (SPF) mice were subjected to either 0 Gy or 5 Gy dose of WBI. At indicated time points, mice were euthanized for cellular analysis. iLN, inguinal lymph node. (B) Representative flow plots of naive (TN), virtual memory (TVM), and true memory CD8+ T cells and Ki67 expression by TN cells in 0 Gy and 5 Gy mice at D25 after WBI. FSC, forward scatter. (C) Number of TN cells per spleen (left) and frequency of Ki67+ TN cells in inguinal lymph node at indicated time points. (D) Number of TVM cells (left) and frequency of TVM cells of total CD8+ T cells per spleen at indicated time points. Data in A–D are representative of 2 independent experiments with n = 4–5 mice per group at each time point. Statistical significance was determined by 2-way ANOVA with Bonferroni’s multiple comparisons post hoc test using GraphPad Prism at each time point. Graphs show the mean ± SEM with each symbol representing 1 mouse. Individual P values are noted on respective graphs or are summarized as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Recovery of TN cells requires de novo thymic output. We next sought to determine the in vivo origin of the emerging TN cells that gradually replenish after WBI. Specifically, we asked the question whether the TN cells that survived the radiation exposure had the capacity to repopulate the TN compartment. To this end, we obtained thymectomized mice that had their thymi removed at 6 weeks of age. These athymic mice allowed us to examine the impact of IR-exposed TN cells versus new thymic emigrants on the numerical recovery of TN cells after WBI. The euthymic and athymic mice were exposed to mock (0 Gy), 2 Gy, and 5 Gy doses of WBI, and the number of TN cells and B cells were longitudinally monitored in the PBL (Figure 3A). It was noted that the 5 Gy dose of WBI was no longer sublethal for the athymic mice, as more than 50% of the mice had died by D36 after WBI. Consistent with previous results, TN cells experienced a dose-dependent numerical decline followed by a gradual recovery in euthymic mice (Figure 3, B and D). However, the number of TN cells in athymic mice stayed low for more than 2 months and never returned to the pre-WBI levels. The sustained loss was specific to TN cells as B cells from both euthymic and athymic mice demonstrated similar kinetics of numerical loss and recovery after WBI (Figure 3, C and E). These data suggest the peripheral TN cells that survive IR exposure cannot adequately repopulate the TN compartment after WBI.
 Figure 3
Figure 3Thymus is required for naive CD8+ T cell numerical recovery. (A) Experimental design: thymectomized and euthymic SPF mice were subjected to 0 Gy, 2 Gy, or 5 Gy dose of WBI and cellular analysis was performed in PBL longitudinally. (B) Number of naive (TN) CD8+ T cells in blood of euthymic (left) and athymic mice (right) at indicated time points. (C) Number of CD19+ B cells in blood of euthymic (left) and athymic mice (right) at indicated time points. Number of TN cells (D) and B cells (E) at each indicated time point was divided to the pre-WBI (D-1) number from the same mouse, and the mean of this ratio was plotted for 2 Gy (left) and 5 Gy (right) euthymic and athymic mice. Data in A–E are representative of 2 independent experiments with n = 2–5 mice per group in each experiment. Statistical significance was determined by 2-way ANOVA with Bonferroni’s multiple comparisons post hoc test. Graphs in B and C show the mean ± SEM, with each symbol representing 1 mouse. Graphs in D and E show the mean ± SD. Individual P values are noted on respective graphs or are summarized as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Previous results highlighted how the TN subset repopulation was impaired in the absence of thymic emigrants after WBI. If the TN subset recovery was mainly mediated by after WBI thymic emigrants, thymic reconstitution should occur prior to numerical recovery of TN cells. Hence, we next investigated the kinetic of loss and recovery of thymocytes following WBI (Supplemental Figure 2A). In agreement with previous reports (47), thymocytes were rapidly lost following a 5 Gy dose of WBI, with the double-positive (DP) thymocytes being the most radiation-sensitive subset and effectively abrogated at D3 after WBI while other subsets were numerically unaltered at this time point. Interestingly, DP cells from 5 Gy hosts were rapidly restored by D10 and demonstrated similar fold change to 0 Gy controls as double-negative, single-positive CD4, and single-positive CD8 thymocytes until full recovery was observed by D60 (Supplemental Figure 2, B–D). The substantial increase in thymocyte numbers occurred between D3 and D10 after WBI, which preceded the marked increase in TN numbers, observed after D10 after WBI. These data indicate that the recovery of TN cells does not occur until the number of thymocytes is sufficiently restored following WBI.
Lymphopenia can induce robust proliferation of CD8+ T cells through HP (48). While IR can induce TN cell death either directly through irreversible DNA damage or indirectly via heightened inflammatory signals and a plethora of cytokines released in response to IR-induced cell death (49, 50), little is known about the fate of the TN cells that survive the initial IR exposure and early IR-driven inflammation. We next asked whether exposure to IR or early IR-mediated inflammation affected the ability of TN cells that survive high doses of IR to undergo HP following RIL. We adoptively transferred Thy1.1+ naive P14 cells, which cannot be de novo–generated by thymopoiesis, to Thy1.2+ hosts, followed by 0 or 5 Gy WBI 2 days later, and assessed the number of endogenous TN and P14 cells at D4 and D34 after WBI (Figure 4, A and B). As expected, the number of P14 and endogenous TN cells did not change between D4 and D34 in 0 Gy hosts. While a significant 15-fold increase in the number of endogenous TN cells occurred in this 30-day period, the number of P14 cells, as a surrogate for IR-experienced TN cells, remained low (Figure 4C). Conversely, when Thy1.1+ naive P14 cells were adoptively transferred once there was an established RIL but P14 cells were not directly exposed to IR (Figure 4D), the number of naive P14 cells increased over time through vigorous cell proliferation similar to the endogenous TN cells (Figure 4, E and F). These data collectively demonstrate that the direct exposure to high dose of IR or the early IR-induced inflammation following WBI impaired the capacity of IR-experienced TN cells to respond to homeostatic cues as the environment after WBI is permissive for proliferation of radiation-inexperienced TN cells.
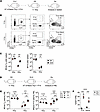 Figure 4
Figure 4WBI-surviving naive CD8+ T cells exhibit diminished proliferative capacity. (A) Experimental design: 106 naive Thy1.1+ P14 CD8+ T cells were adoptively transferred into Thy1.2+ naive hosts followed by exposure to 0 Gy or 5 Gy dose of WBI 2 days later. Spleens were harvested and analyzed at indicated time points. (B) Representative flow plots of P14 CD8+ T cells and endogenous naive (TN) and virtual memory (TVM) CD8+ T cells in 0 Gy and 5 Gy mice at D34 after WBI. (C) Number of endogenous TN cells (left) and P14 cells (right) in 0 Gy and 5 Gy hosts at indicated time points after WBI. (D) Experimental design: 7.5 × 105 naive P14 CD8+ T cells were adoptively transferred to Thy1.2+ naive hosts that had undergone 0 Gy or 5 Gy WBI 2 days prior. Longitudinal blood analysis was performed at indicated time points. (E) Number of endogenous TN cells (left) and naive P14 cells (right) in 0 Gy and 5 Gy hosts at indicated time points after WBI. (F) Frequency of P14 and TN cells that express Ki67 in 0 Gy and 5 Gy hosts at D10 after WBI. Data in A–F are representative of 2 independent experiments with n = 5–8 mice per group in each experiment. Statistical significance was determined by 2-way ANOVA with Bonferroni’s multiple comparisons post hoc test using GraphPad Prism. Graphs show the mean ± SEM with each symbol representing 1 mouse. Individual P values are noted on respective graphs or are summarized as follows: **P < 0.01, ***P < 0.001.
After recovery TN cells display altered phenotypic and clonal composition. Thus far we have investigated the numerical changes to TN cells following WBI and the factors that influence the efficacy of the after WBI TN reconstitution. We next turned our focus to assessing the qualitative changes that may also occur to the composition of TN cells following recovery from WBI. Recent studies have demonstrated the impact of homeostatic cues in shaping the phenotypic and functional heterogeneity of the TN population. Specifically, Ly6C expression demarcates a subpopulation of TN cells with enhanced cognate Ag-mediated effector function and Ag-independent bystander responses (51–53). We sought to define any potential changes to the phenotype of TN cells throughout the recovery process after WBI (Figure 5A). D30 TN cells were clustered based on expression of Ly6C, CD122, CD44, CD62L, CD127, CD11a, and CD49d using FlowSOM, and these clusters were projected into t-distributed stochastic neighbor embedding (t-SNE) plots for further analysis (Figure 5B). Although clusters 1 and 2 together accounted for the majority of the naive CD8+ T cells in both groups, cluster 1 was more enriched within 5 Gy TN cells whereas cluster 2 was more abundant within 0 Gy TN cells (Figure 5, B and C). Both clusters similarly expressed CD127 and CD62L, 2 canonical markers of TN cells; however, Ly6C and CD122 expression was significantly higher in cells within cluster 2, suggesting the representation of Ly6C+CD122+ TN cells was increased in 5 Gy mice (Figure 5D). Our longitudinal PBL analysis of the frequency of Ly6C+ and Ly6C+CD122+ TN cells revealed the enrichment of these subpopulations lasts up to D60 after WBI and was not due to preferential post-WBI survival (Figure 5, E and F). These findings showed the post-WBI TN subset undergoes lasting phenotypic alterations.
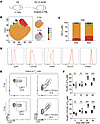 Figure 5
Figure 5Phenotypic composition of TN cells undergoes lasting alterations following numerical recovery from WBI. (A) Experimental design: SPF mice were subjected to 0 Gy or 5 Gy dose of WBI, and cellular analysis was performed in PBL longitudinally. (B) Left: t-SNE analysis of PBL displaying FlowSOM defined clusters among D30 naive (TN: CD44loCD8α+) CD8+ T cells based on expression of CD8α, CD11a, CD44, CD62L, CD49d, Ly6C, CD127, CD122. Right: clusters that are most robustly enriched in 0 Gy (top) and 5 Gy (bottom) groups. (C) Cluster distribution among TN cells of 0 Gy and 5 Gy mice. (D) Histograms of Ly6C, CD122, CD44, CD127, and CD62L surface expression comparing clusters 1 and 2. (E) Representative flow plots and (F) frequency of Ly6C+ and Ly6C+CD122+ TN cells at indicated time points after WBI. Data in A–F are representative of 2 independent experiments with n = 5–7 mice per group in each experiment. Statistical significance was determined by 2-way ANOVA with Bonferroni’s multiple comparisons post hoc test using GraphPad Prism. Graphs show the mean ± SEM with each symbol representing 1 mouse. Individual P values are noted on respective graphs or are summarized as follows: ***P < 0.001.
Given the propensity of TN cells with higher affinity for self-peptide–MHC ligands to undergo HP following RIL than low-affinity TN cells, we next investigated to what extent the clonal diversity of the TN subset changes once numerical recovery is achieved. To this end, TCRβ variable chain isotype frequencies within the TN population of 0 Gy and 5 Gy mice were compared early (D3) and late (D60) after WBI (Supplemental Figure 3, A and B). Importantly, the TCR Vβ profile of 0 Gy splenic TN cells examined at 4 time points indicated a very stable pool with little to no change to the “status quo” TN diversity within this period (Supplemental Figure 3C). This assessment enabled us to determine whether TN cells expressing distinct Vβ chains were differentially lost (D3) or recovered (D60) following WBI. While the frequency of cells expressing Vβ3, Vβ5.1/5.2, Vβ6, and Vβ10b did not change after the number of TN cells acutely reduced, Vβ6 and Vβ5.1/5.2 cells were differentially recovered, leading to the underrepresentation of the former and overrepresentation of the latter population at D60 after WBI. Additionally, TN cells expressing Vβ2, Vβ8.1 /8.2, Vβ8.3, Vβ11, Vβ13, and Vβ14 were all differentially susceptible to WBI-induced loss, and the frequency of Vβ8.1/8.2 and Vβ13 expressing cells still remained lower than 0 Gy counterparts 60 days after WBI (Supplemental Figure 3D). These data collectively indicated the altered clonal diversity within the TN repertoire after recovery from WBI.
Both newly emerging and radiation-survived naive CD8+ T cells can differentiate into TVM cells. Our data in Figures 1 and 2 show the reconstitution of the bulk TN population was concomitant with reconstitution and transient overrepresentation of the TVM population. This observation prompted us to explore the potential for distinct changes within individual Ag-specific CD8+ T cell populations with respect to their numerical recovery and phenotypic change following WBI. Using a p:MHC I tetramer-based enrichment method (31, 54), we analyzed the precursor frequency and phenotype of 4 endogenous CD8+ T populations specific for ovalbumin (OVA257-264), LCMV-derived GP33-41, vaccinia virus-derived B8R20-27, and Plasmodium-derived GAP5040-48 epitopes in SPF hosts that underwent 0 or 5 Gy dose of WBI 28 days prior (Figure 6, A–C). Precursor numbers in 0 Gy hosts were consistent with previous analyses that reported various population sizes for each Ag-specific population with GAP5040-48–specific CD8+ T cells constituting the largest pool of Ag-specific cells followed by B8R20-27–, GP33-41–, and OVA257-264–specific repertoires (55, 56) (Figure 6D). Importantly, all 4 populations from 5 Gy mice showed a similar drop in population size compared to the 0 Gy mice. Additionally, phenotypic analysis of the populations with enough recovered cells (3 out of 4) showed an increased fraction of cells with TVM phenotype, suggesting that following WBI, TN cells can acquire a TVM phenotype independent of their Ag specificity (Figure 6E). To formally show the capacity of TN cells to give rise to TVM progeny following WBI, we analyzed the number of P14 cells with TVM phenotype following transfer of naive P14 cells 2 days after 0 or 5 Gy WBI (Figure 6F). In 5 Gy recipients, endogenous TVM cells and P14 cells with a TVM phenotype gradually increased in number over time, whereas their levels remained stable in 0 Gy recipients (Figure 6G). These data suggest that radiation-inexperienced TN cells can acquire a TVM phenotype following RIL.
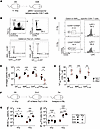 Figure 6
Figure 6Acquisition of virtual memory phenotype by a fraction of proliferating TN cells after WBI. (A) Experimental design: Thy1.2+ hosts were subjected to 0 Gy or 5 Gy dose of WBI. At 28 days later, the number and phenotype of Ag-specific CD8+ T cells specific for GP33-41, B8R20-27, GAP41-48, and OVA257-264 were determined using pMHC class I tetramer-based enrichment from spleens. (B) Representative flow plots showing gating strategy used to detect Ag-specific CD8+ T cell precursors and (C) virtual memory phenotype (CD44hiCD49loLy6C+) from Ag-specific CD8+ T cell precursors in 0 Gy and 5 Gy mice. (D) Absolute number and (E) frequency of virtual memory phenotype of Db-restricted GP33-41– and GAP5040-48–specific and Kb-restricted B8R20-27– and OVA257-264–specific CD8+ T cells. (F) Experimental design: 7.5 × 105 naive P14 CD8+ T cells were adoptively transferred to Thy1.2+ naive hosts that had undergone 0 Gy or 5 Gy WBI 2 days prior. Longitudinal blood analysis was performed at indicated time points. (G) Number of endogenous (left) and P14 (right) virtual memory (TVM) CD8+ T cells in 0 Gy and 5 Gy hosts at indicated time points after WBI. Data in A–E are from a single experiment, and F and G are representative of 2 independent experiments with n = 5–8 mice per group in each experiment. Statistical significance was determined by unpaired multiple t tests with Holm-Šídák multiple-comparison post hoc test or 2-way ANOVA with Bonferroni’s multiple comparisons post hoc test using GraphPad Prism. Graphs show the mean ± SEM with each symbol representing 1 mouse. Individual P values are noted on respective graphs or are summarized as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Given the capacity of radiation-inexperienced TN and TVM cells to generate TVM cells, we last asked whether TN and TVM cells that survived IR exposure can give rise to more TVM cells following WBI (Figure 7A). To this end, we tracked the number of TVM cells in PBL after euthymic and athymic mice were exposed to 0, 2, and 5 Gy doses of WBI. Interestingly, both euthymic and athymic mice were able to reconstitute their TVM compartment to pre-WBI numbers (Figure 7, B and D), which was in sharp contrast with the diminished repopulation of TN cells in athymic mice (Figure 7, B and D). Although the gradual repopulation of TVM cells contributed to an increase in total CD8+ T cell numbers of both euthymic and athymic mice (Figure 7C), this was not sufficient to fully restore the CD8+ T cell numbers to the pre-WBI baseline in athymic mice (Figure 7E). These data collectively suggest that TVM cells can arise from radiation-experienced TN and TVM cells.
 Figure 7
Figure 7Repopulation of the virtual memory subset can occur in the absence of thymus. (A) Experimental design: same as Figure 3A. (B) Number of virtual memory (TVM) CD8+ T cells in blood of euthymic (left) and athymic mice (right) at indicated time points. (C) Number of total CD8+ T cells in blood of euthymic (left) and athymic mice (right) at indicated time points. Number of TVM (D) and total CD8+ T cells (E) at each indicated time point was divided to the pre-WBI (D-1) number from the same mouse, and the mean ± SD of this ratio was plotted for 2 Gy (left) and 5 Gy (right) euthymic and athymic mice. Data in A–E are representative of 2 independent experiments with n = 2–5 mice per group in each experiment. Statistical significance was determined by 2-way ANOVA with Bonferroni’s multiple comparisons post hoc test. Graphs in B and C show the mean ± SEM with each symbol representing 1 mouse. Graphs in D and E show the mean ± SD. Individual P values are noted on respective graphs or are summarized as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
-
Discussion
Patients who receive high doses of IR as part of their solid tumor treatment or prior to hematopoietic stem cell transplant often experience a sustained lymphopenia that can vary greatly in duration (57). In addition to quantitative recovery, functional reconstitution of T cells also depends on balanced recovery of different subsets of T cells and TCR diversity (35). While T cell repopulation following WBI has been attributed to both HP of IR-surviving cells and T cell neogenesis in thymus, here we investigated the contribution of each mechanism to the reconstitution of Ag-inexperienced CD8+ T cells. We also investigated the short- and long-term effects of sublethal WBI on the phenotype and diversity of Ag-inexperienced CD8+ T cells. Our data support the notion that following IR-induced global loss of both TN and TVM cells, gradual numerical recovery of both subsets occurs, with the TVM subset temporarily being enriched during early stages of lymphocyte recovery. While thymopoiesis is critical for efficient remission of TN cells, the post-recovery TN compartment may not fully recapitulate pre-WBI TCR diversity. Nevertheless, the TN subset is never restored in the absence of the thymus, and only delayed TVM recovery contributes to partial numerical recovery of the CD8+ T cell compartment.
We have previously demonstrated the inability of circulating and tissue-resident TMEM cells to numerically increase following WBI (41, 58). Similarly, our results indicated the number of TN cells that survived IR exposure remain low in euthymic mice, suggesting the contribution of such cells to the recovering TN and TVM subset is limited. Therefore, after WBI thymic neogenesis is required for effective regeneration of the TN subset while differentiation of some of the newly generated thymic emigrants or mature TN cells to a TVM phenotype restores the TVM subset in euthymic mice. Interestingly, athymic SPF mice exhibited a delayed but complete numerical recovery of the TVM subset after WBI. Although the numerical expansion of IR-experienced TN (or TVM) cells in athymic mice may look contradictory to the limited proliferative capacity of IR-experienced TN cells in euthymic mice, one could postulate the new thymic emigrants that enter the lymphopenic environment after WBI outcompete the IR-experienced CD8+ T cells in sensing signals that drive HP and dominate the reconstitution of TN and TVM compartments. Therefore, it is only in the instance of thymic absence that IR-experienced CD8+ T cells receive adequate levels of homeostatic stimuli to substantially proliferate. Whether IR-experienced TN and/or TVM cells contribute to the TVM subset recovery in athymic mice remains to be determined.
Although thymus-derived CD8+ T cells are instrumental in reconstituting the TN subset after WBI, phenotypic and TCR clonotype composition of TN cells is altered after numerical recovery. Altered TCR diversity among TN cells may stem from differential capacity of the newly generated TN cells to undergo HP in the lymphopenic environment after WBI, leading to changes to the composition of the TCR repertoire. Additionally, we noted an increase in the population of Ly6C+ TN cells for up to 60 days in 5 Gy hosts. Ly6C expression is induced by type I interferon (IFN) signaling, which is upregulated shortly after many intracellular infections, microbial exposure through cohousing, and polymicrobial sepsis (53, 59). We speculate the IR-induced increase in type I IFN (60, 61) in conjunction with increased TCR contact with self-pMHC because of prolonged lymphopenia promotes Ly6C upregulation on the newly emerging TN cells. Additionally, Ly6C+ TN cells proliferate vigorously in the lymphopenic environment, which can further lead to an enrichment of Ly6C+ TN cells after WBI. Ly6C+ TN cells are endowed with a greater effector and memory formation capacity and bystander activation than Ly6C– TN cells (52, 59). This enrichment may allow for a more robust effector response in hosts that are still recovering from RIL and hence confer advantage in combating new and recurring pathogens.
We provide evidence that TVM phenotype acquisition by Ag-specific CD8+ T cells occurs independent of their specificity in euthymic mice. The rapid recovery of the TVM subset in irradiated euthymic mice is reminiscent of emergence of phenotypically similar HP memory CD8+ T cells that arise after vigorous expansion and memory marker acquisition of TN cells upon adoptive transfer to sublethally irradiated mice (62–64). While TVM and HP memory CD8+ T cells both share memory markers, whether both represent the same population is not well understood, as they arise under different homeostatic circumstances. It is crucial to investigate whether the propensity of after WBI TVM cells in differentiating to Ag-specific effector and true memory cells is any different than the TVM cells in nonirradiated mice. Additionally, as IR exposure impairs the cognate Ag-derived effector function of TMEM cells, it is plausible that IR-experienced TN and TVM cells have reduced per-cell capacity to give rise to Ag-specific effector and true memory cells, but this hypothesis remains to be investigated.
Human TVM cells have been identified as CD45RA+CCR7–CD8+ T cells that express KIR/NKG2A (28, 65). Similar to the pool of TMEM cells, TVM cells accumulate with age while the thymic output and TN pool size progressively diminish (28, 66, 67). An important finding of the current study is the elevated proportions of TVM cells within the CD8+ T cell pool in mice with history of pathogen exposure and athymic mice following WBI and subsequent CD8+ T cell reconstitution. Unlike in SPF mice, the overrepresentation of TVM cells in LCMV-immune mice was likely due to the WBI-mediated permanent loss of approximately 80% of pathogen-specific circulatory memory CD8+ T cells while Ag-inexperienced CD8+ T cells gradually repopulated and established the pre-WBI numbers. In addition, only the TVM subset was fully restored in athymic SPF mice while the number of TN cells stalled. Using classic gating (CD45RA vs. CCR7), human TVM cells fall within a larger subset of CD8+ T cells termed as terminal effector memory CD8+ T cells that expand in patients recovering from allogeneic hematopoietic stem cell transplant (68). Our findings may suggest that in individuals recovering from an episode of severe lymphopenia, the TVM subset may constitute a greater fraction of total CD8+ T cells than previously appreciated and may warrant further investigation.
Differences in murine and human lymphocyte response to IR and RIL may limit the extent to which the findings of this study can be extended. The relatively expeditious numerical recovery of lymphocytes in adult mice following WBI-mediated or focal brain irradiation–induced RIL does not recapitulate the prolonged RIL episodes that are often seen in patients who underwent radiotherapy (69). In addition, previous studies have pointed to differential gene expression patterns in mouse and human blood cells following exposure to high doses of IR, which may influence the fate of IR-experienced CD8+ T cells in both species (70). Notably, blood cells from hematopoietically humanized mice display IR-induced gene expression profiles more similar to those of human WBI blood samples than those of murine blood cells (70). Hence, it would be of interest to investigate the dynamic changes to the number and phenotype of CD8+ T cell subsets in hematopoietically humanized mice following IR exposure.
In summary, this study provides an overview of the dynamic changes to the Ag-inexperienced CD8+ T cell subset following WBI. Our findings emphasize the indisputable role of thymus in TN regeneration while TVM recovery can be achieved in the absence of thymopoiesis. While we documented lasting changes to the subset representation and TCR diversity of TN cells after WBI, future studies on functional implications of such alterations will inform therapeutic approaches to address lymphopenia and the immune incompetence associated with it.
-
Methods
Sex as a biological variable. Experiments were conducted using both male and female mice, with no differences observed in the kinetic of loss, recovery, and phenotypic changes to the bulk Ag-inexperienced CD8+ T cells following WBI. Thereafter, our experimental designs involving adoptive transfer of transgenic CD8+ T cells, thymectomized mice, and MHC class I tetramer-based enrichment utilized female mice, with the findings expected to be relevant for all sexes.
Mice, infections, and memory CD8+ T cell generation. Inbred C57BL/6 (Thy 1.2/1.2) mice were purchased from Charles River and maintained in the animal facilities at the University of Iowa at the appropriate biosafety level. Thymectomized C57BL/6 mice were purchased from Jackson Laboratory. P14 TCR-transgenic mice (Thy1.1/1.1) were bred and maintained at the University of Iowa (Iowa City, Iowa, USA).
To generate memory CD8+ T cells, 104 naive P14 TCR-Tg CD8+ T cells were adoptively transferred into C57BL/6 mice, followed by infection with 2 × 105 PFU of LCMV-Armstrong by i.p. injection a day later. In experiments with adoptive transfer of naive P14 CD8+ T cells but no subsequent LCMV infection, 0.75 × 106 to 1.0 × 106 naive cells were transferred.
Irradiation. WBI was performed by placing the mice in a 225 kv rotating x-ray tube (Small Animal Radiation Research platform; Xstrahl) available at the Radiation Core at the University of Iowa. Radiation doses of 2 Gy and 5 Gy were used in this study. Mock-treated mice were placed into the same mouse pie cages as IR-treated mice were and remained there for the length of a 5 Gy exposure without WBI exposure.
Cell isolation. Peripheral blood was collected by retro-orbital bleeding. Single-cell suspensions from spleen and lymph nodes were generated after mashing tissue through a 70 μm cell strainer (Corning) without enzymatic digestion. ACK lysis buffer was used for red blood cell lysis from peripheral blood and spleen samples.
Cell staining and flow cytometry. Single-cell suspensions were incubated with a cocktail of fluorescently labeled mAbs for 30 minutes at 4°C. These mAbs included: CD8a 53-6.7, BioLegend), CD11a (M17/4, BioLegend), Thy1.1 (OX7, BioLegend), CD49d (R1-2, BioLegend), CD62L (MEL14, BioLegend), CD44 (IM7, BioLegend), CD4 (GK1.5, BioLegend), CD19 (1D3, BioLegend), Vβ2 (B20.6, BioLegend), Vβ3 (KJ25, BD), Vβ5.1/5.2 (MR9-4, BioLegend), Vβ6 (RR4-7, eBioscience), Vβ8.1/8.2 (KJ16-133, eBioscience), Vβ8.3 (1B3.3, BioLegend), Vβ10b (B21.5, BioLegend), Vβ11 (KT11, BioLegend), Vβ13 (MR12-3, eBioscience), Vβ14 (14-2, BD), Ly6C (HK1.4, BioLegend) CD122 (5H4, BioLegend), and CD127 (eBioSB/199, eBioscience). Samples were then fixed with Cytofix (BD Biosciences) at 4°C for 15 minutes. To stain for Ki-67 (B56, BD Biosciences), Foxp3/Transcription Factor Staining Buffer reagents (eBioscience) and protocol were used. Flow cytometry data were acquired using an LSRFortessa (BD Biosciences) and analyzed using FlowJo software v.10 (FlowJo LLC) using FlowSOM and tSNE plug-ins.
Enrichment and characterization of endogenous Ag-specific naive CD8+ T cells. Tetramer-based enrichment protocol (31, 71) using GP33-41 and GAP5040-48 containing Db MHC class I tetramer and OVA257-264 and B8R20-27 containing Kb MHC class I tetramers was used. In brief, single-cell suspension from splenocytes was stained with PE- and APC-conjugated pMHC I tetramers in 300 μL tetramer staining buffer (PBS containing 5% FBS, 2 mM EDTA, 1:50 normal mouse serum, and 1:100 anti-CD16/32 mAb). The cells were incubated in the dark at room temperature for 1 hour, followed by a wash in 10 mL ice-cold FACS Buffer. The tetramer-stained cells were then resuspended in 300 μL FACS Buffer, mixed with 25 μL of anti-PE and anti-APC mAb-conjugated magnetic microbeads (STEMCELL Technologies), and incubated in the dark on ice for 30 minutes. The cells were washed, resuspended in 3 mL cold FACS Buffer, and passed through an EasySep Magnet (STEMCELL Technologies) to yield the enriched tetramer-positive population. The resulting enriched fractions were stained with a cocktail of fluorochrome-labeled mAbs: Thy1.2 (53–2.1, eBioscience), CD4, CD8, CD44, CD49d, Ly6C, and “dump/live” (CD11b [M1/70, eBioscience], CD11c [N418, eBioscience], B220 [RA3-6B2, eBioscience], F4/80 [BM8, eBioscience], Live/Dead [eBioscience]). Cell numbers for each sample were determined using AccuCheck Counting Beads (Invitrogen). Samples were then analyzed using a Fortessa flow cytometer (BD Biosciences) and FlowJo software (TreeStar).
Statistics. All statistical analyses were performed using GraphPad Prism (v10.0). When indicated, 2-tailed unpaired Student’s t tests were performed when comparing 2 independent groups and 2-way ANOVA with Bonferroni’s multiple comparisons test when comparing more than 2 groups for more than 1 variable. P values are indicated in individual figures or in figure legends. P < 0.05 was considered significant.
Study approval. Experimental procedures using mice were approved by the University of Iowa Animal Care and Use Committee under protocol numbers 2121915.
Data availability. All data associated with this study can be found in the main text or the supplement. Values for all data points in graphs are reported in the Supporting Data Values files.
-
Author contributions
MH, JTH, and VPB conceived and the designed the study. MH, SKK, and WS conducted experiments and analyzed data. MH and VPB wrote the original draft, and SKK, VPB, TSG, and JTH reviewed and edited the manuscript. TSG, JTH, and VPB acquired funding and supervised the project.
-
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants R01AI114543 (to VPB and JTH), R01AI042767 (to JTH), and GM140881 (to TSG) and a Veterans Administration Merit Review Award (BX001324 to TSG). TSG is the recipient of a Research Career Scientist award (IK6BX006192) from the Department of Veterans Affairs. VPB is a University of Iowa Distinguished Scholar. We thank members of the Harty and Badovinac labs for valuable discussions. We also thank Vahid Nasirian at Ionizing Radiation Services at Free Radical Research Core for assisting with WBI of mice. This work is the result of NIH funding, in whole or in part, and is subject to the NIH Public Access Policy. Through acceptance of this federal funding, the NIH has been given a right to make the work publicly available in PubMed Central.
Address correspondence to: Vladimir P. Badovinac, Department of Pathology, The University of Iowa, 1020 Medical Laboratories, 25 S. Grand Avenue, Iowa City, Iowa 52246, USA. Phone: 319.384.2930; Email: Vladimir-badovinac@uiowa.edu.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2025, Heidarian et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2025;10(18):e194201.https://doi.org/10.1172/jci.insight.194201.
-
References
- Sia J, et al. Molecular mechanisms of radiation-induced cancer cell death: a primer. Front Cell Dev Biol. 2020;8:41.
- Rodríguez-Ruiz ME, et al. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–655.
- Vanpouille-Box C, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8(1):15618.
- Giuranno L, et al. Radiation-induced lung injury (RILI). Front Oncol. 2019;9:877.
- Straub JM, et al. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–1994.
- Henson CC, et al. Structured gastroenterological intervention and improved outcome for patients with chronic gastrointestinal symptoms following pelvic radiotherapy. Support Care Cancer. 2013;21(8):2255–2265.
- Heylmann D, et al. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014;1846(1):121–129.
- Heylmann D, et al. Sensitivity of CD3/CD28-stimulated versus non-stimulated lymphocytes to ionizing radiation and genotoxic anticancer drugs: key role of ATM in the differential radiation response. Cell Death Dis. 2018;9(11):1053.
- Nakamura N, et al. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123(2):224–227.
- Terrones-Campos C, et al. Hematological toxicity in patients with solid malignant tumors treated with radiation - temporal analysis, dose response and impact on survival. Radiother Oncol. 2021;158:175–183.
- Damen PJ, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2021;111(4):936–948.
- Venkatesulu BP, et al. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51.
- Ménétrier-Caux C, et al. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer. 2019;7(1):85.
- Pike LR, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2019;103(1):142–151.
- Venkatesulu B, et al. Lymphocyte sparing normal tissue effects in the clinic (LymphoTEC): a systematic review of dose constraint considerations to mitigate radiation-related lymphopenia in the era of immunotherapy. Radiother Oncol. 2022;177:81–94.
- Tubin S, et al. Mono-institutional phase 2 study of innovative Stereotactic Body RadioTherapy targeting PArtial Tumor HYpoxic (SBRT-PATHY) clonogenic cells in unresectable bulky non-small cell lung cancer: profound non-targeted effects by sparing peri-tumoral immune microenvironment. Radiat Oncol. 2019;14(1):212.
- Adamo S, et al. Signature of long-lived memory CD8+ T cells in acute SARS-CoV-2 infection. Nature. 2022;602(7895):148–155.
- Schmidt ME, Varga SM. The CD8 T cell response to respiratory virus infections. Front Immunol. 2018;9:678.
- Sahin U, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226.
- Badovinac VP, et al. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3(7):619–626.
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8(2):107–119.
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9(12):823–832.
- Youngblood B, et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552(7685):404–409.
- Tanchot C, et al. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276(5321):2057–2062.
- Nešić D, Vukmanovic S. MHC class I is required for peripheral accumulation of CD8+ thymic emigrants. J Immunol. 1998;160(8):3705–3712.
- Vivien L, et al. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int Immunol. 2001;13(6):763–768.
- Haluszczak C, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206(2):435–448.
- White JT, et al. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun. 2016;7(1):11291.
- Drobek A, et al. Strong homeostatic TCR signals induce formation of self-tolerant virtual memory CD8 T cells. EMBO J. 2018;37(14):e98518.
- Cabrera-Perez J, et al. Alterations in antigen-specific naive CD4 T cell precursors after sepsis impairs their responsiveness to pathogen challenge. J Immunol. 2015;194(4):1609–1620.
- Condotta SA, et al. Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J Immunol. 2013;190(5):1991–2000.
- Slütter B, et al. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol. 2017;2(7):eaag2031.
- Nolz Jeffrey C, Harty John T. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34(5):781–793.
- Mackall CL, et al. Restoration of T-cell homeostasis after T-cell depletion Semin Immunol. 1997;9(6):339–346.
- Velardi E, et al. T cell regeneration after immunological injury. Nat Rev Immunol. 2021;21(5):277–291.
- Mackall CL, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332(3):143–149.
- Mackall CL, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89(10):3700–3707.
- den Braber I, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36(2):288–297.
- Qi Q, et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A. 2014;111(36):13139–13144.
- Uhlemann H, et al. Shape of the art: TCR-repertoire after allogeneic hematopoietic cell transplantation. Best Pract Res Clin Haematol. 2024;37(2):101558.
- Heidarian M, et al. Sublethal whole-body irradiation induces permanent loss and dysfunction in pathogen-specific circulating memory CD8 T cell populations. Proc Natl Acad Sci U S A. 2023;120(27):e2302785120.
- Rai D, et al. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183(12):7672–7681.
- Martin MD, et al. Revealing the complexity in CD8 T cell responses to infection in inbred C57B/6 versus outbred Swiss mice. Front Immunol. 2017;8:1527.
- Chiu BC, et al. Cutting edge: central memory CD8 T cells in aged mice are virtual memory cells. J Immunol. 2013;191(12):5793–5796.
- Berton RR, et al. Accurate enumeration of pathogen-specific and virtual memory CD8 T cells after infection. J Immunol. 2025;214(5):995–1007.
- White JT, et al. Antigen-inexperienced memory CD8+ T cells: where they come from and why we need them. Nat Rev Immunol. 2017;17(6):391–400.
- Pan B, et al. Acute ablation of DP thymocytes induces up-regulation of IL-22 and Foxn1 in TECs. Clin Immunol. 2014;150(1):101–108.
- Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11(2):183–190.
- Jiang J, et al. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol. 2003;171(8):4352–4358.
- Cytlak UM, et al. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. 2022;22(2):124–138.
- Fulton RB, et al. The TCR’s sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat Immunol. 2015;16(1):107–117.
- Ju Y-J, et al. Self-reactivity controls functional diversity of naive CD8+ T cells by co-opting tonic type I interferon. Nat Commun. 2021;12(1):6059.
- Jergović M, et al. Infection-induced type I interferons critically modulate the homeostasis and function of CD8+ naïve T cells. Nat Commun. 2021;12(1):5303.
- Obar JJ, et al. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28(6):859–869.
- Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012;188(9):4135–4140.
- Van Braeckel-Budimir N, et al. A T cell receptor locus harbors a malaria-specific immune response gene. Immunity. 2017;47(5):835–847.
- De Kermenguy F, et al. Radio-induced lymphopenia in the era of anti-cancer immunotherapy. Int Rev Cell Mol Biol. 2023;378:1–30.
- Hassert M, et al. Regenerating murine CD8+ lung tissue resident memory T cells after targeted radiation exposure. J Exp Med. 2024;221(3):e20231144.
- Berton RR, et al. Sepsis leads to lasting changes in phenotype and function of naïve CD8 T cells. PLoS Pathog. 2023;19(10):e1011720.
- Leibowitz BJ, et al. Interferon b drives intestinal regeneration after radiation. Sci Adv. 2021;7(41):eabi5253.
- Feng X, et al. ATR inhibition potentiates ionizing radiation-induced interferon response via cytosolic nucleic acid-sensing pathways. EMBO J. 2020;39(14):e104036.
- Cho BK, et al. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192(4):549–556.
- Goldrath AW, et al. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192(4):557–564.
- Hamilton SE, et al. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7(5):475–481.
- Jacomet F, et al. Evidence for eomesodermin-expressing innate-like CD8(+) KIR/NKG2A(+) T cells in human adults and cord blood samples. Eur J Immunol. 2015;45(7):1926–1933.
- Geginat J, et al. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101(11):4260–4266.
- Quinn KM, et al. Age-related decline in primary CD8+ T cell responses is associated with the development of senescence in virtual memory CD8+ T cells. Cell Rep. 2018;23(12):3512–3524.
- Alho AC, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127(5):646–657.
- Piotrowski AF, et al. Systemic depletion of lymphocytes following focal radiation to the brain in a murine model. Oncoimmunology. 2018;7(7):e1445951.
- Ghandhi SA, et al. Discordant gene responses to radiation in humans and mice and the role of hematopoietically humanized mice in the search for radiation biomarkers. Sci Rep. 2019;9(1):19434.
- Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27(2):203–213.
-
Version history
- Version 1 (August 5, 2025): In-Press Preview
- Version 2 (September 23, 2025): Electronic publication










