Abstract
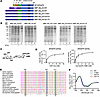
Ehlers-Danlos syndromes (EDS) comprise a genetically and clinically heterogenous group of rare diseases that cause severe, often fatal, damage to connective tissue. The molecular basis of EDS implicates defects in extracellular matrix components, including various fibrillar collagens and glycosaminoglycans (GAGs). However, the precise pathogenic mechanisms behind EDS remain elusive. Here, we have implemented a multi-tiered approach to demonstrate the functional impact of B3GALT6 mutations on biochemical and developmental processes, ultimately leading to the spondylodysplastic subtype of EDS (spEDS), characterized by severe musculoskeletal symptoms. We show that the loss of function of β1,3-galactosyltransferase 6 (β3GalT6) is partially compensated by β1,3-glucuronosyltransferase 3 (GlcAT-I), the next enzyme in the GAG biosynthetic pathway. In addition, results from transcriptomics, collagen analysis, and biophysical experiments revealed that impaired collagen maturation, including defective glycosylation of collagen XII, contributes to altered tissue structure and biomechanics, the hallmarks of spEDS. Our findings unravel a new pathogenic mechanism of spEDS and bring us one step closer to therapeutic strategies, including cell and tissue engineering.
Authors
Roméo Milan Diana, Benjamin Jolivet, Jean-Baptiste Vincourt, Sébastien Hergalant, Grégory Francius, Yasaman Karami, Hamed Khakzad, Rebekka Wild, Marie Bourgeais, Anne Robert, Alison Wurtz, Guillermo Barreto, Nick Ramalanjaona, Déborah Helle, Rachel Onifarasoaniaina, Sophie Front, Chrystel Lopin-Bon, Delfien Syx, Fransiska Malfait, Sylvie Fournel-Gigleux, Sandrine Gulberti, Catherine Bui
Abstract
Abdominal aortic aneurysm (AAA) is a life-threatening vascular disease with no effective pharmacological interventions. While single-cell transcriptomics has advanced our understanding of AAA, it lacks spatial context. Here, we employed Seq-Scope, an ultra-high-resolution spatial transcriptomic technology, to decipher the spatial landscape of angiotensin II–induced AAA in Apoe–/– mice. Our analysis revealed the heterogeneity of macrophages, fibroblasts, and smooth muscle cells (SMCs), with specific responses in different layers of the AAA tissue. SMCs in the inner layers showed associations with Mgp-expressing fibroblasts and GPNMB-expressing macrophages, whereas the outer layers had different dominant cell types. Notably, GPNMB-expressing macrophages were concentrated near SMCs in regions of severe elastic lamina damage. Immunofluorescent staining confirmed their colocalization, and scRNA-seq reanalysis independently validated the presence of GPNMB-high macrophages in AAA tissues, highlighting their involvement in inflammation and tissue remodeling. Moreover, we discovered that macrophage-derived soluble GPNMB induces SMC phenotypic switching, reducing contractile markers while increasing cytokines and metalloproteinases. This effect was partly mediated by CD44 signaling. These findings suggest that GPNMB-high macrophages contribute to AAA development by driving SMC dysfunction. This study highlights the importance of high-resolution spatial transcriptomics in complementing single-cell transcriptomics, offering valuable insights into molecular and cellular responses in the AAA microenvironment.
Authors
Guizhen Zhao, Chun-Seok Cho, Hongyu Liu, Yongha Hwang, Yichen Si, Myungjin Kim, Yongjie Deng, Yang Zhao, Chao Xue, Yanhong Guo, Lin Chang, Dogukan Mizrak, Bo Yang, Hyun Min Kang, Jifeng Zhang, Jun Hee Lee, Y. Eugene Chen
Abstract
Pulmonary fibrosis (PF) is a life-threatening disease that requires effective and well-tolerated therapeutic modalities. Previously, the distinct pathogenic roles of cannabinoid receptor 1 (CB1R) and inducible nitric oxide synthase (iNOS) in the lungs and their joint therapeutic targeting were highlighted in PF. However, the cell-specific role of CB1R in PF has not been explored. Here, we demonstrate that CB1R in alveolar macrophages (AMs) mediates the release of anandamide into the alveoli, which promotes PF by inducing pro-fibrotic macrophages that are accessible to locally delivered antifibrotic therapy. A multitargeted therapy may improve therapeutic efficacy in PF. Pulmonary delivery of 0.5 mg/kg/d MRI-1867 (zevaquenabant), a peripherally acting hybrid CB1R/iNOS inhibitor, was as effective as systemic delivery of 10 mg/kg/d and also matched the efficacy of nintedanib in mitigating bleomycin-induced PF. A systems pharmacology approach revealed that zevaquenabant and nintedanib treatments reversed pathologic changes in both distinct and shared PF-related pathways, which are conserved in human and mouse. Moreover, zevaquenabant treatment also attenuated fibrosis and pro-fibrotic mediators in human precision-cut lung slices. These findings establish CB1R-expressing AMs as a therapeutic target and support local delivery of dual CB1R/iNOS inhibitor zevaquenabant by inhalation as an effective, well-tolerated, and safe strategy for PF.
Authors
Abhishek Basu, Muhammad Arif, Kaelin M. Wolf, Madeline Behee, Natalie Johnson, Lenny Pommerolle, Ricardo H. Pineda, John Sembrat, Charles N. Zawatsky, Szabolcs Dvorácskó, Nathan J. Coffey, Joshua K. Park, Seray B. Karagoz, Grzegorz Godlewski, Tony Jourdan, Judith Harvey-White, Melanie Königshoff, Malliga R. Iyer, Resat Cinar
Abstract
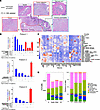
Pancreatic ductal adenocarcinoma (PDAC) has a poor survival rate due to late detection. PDAC arises from precursor microscopic lesions, termed pancreatic intraepithelial neoplasia (PanIN), that develop at least a decade before overt disease; this provides an opportunity to intercept PanIN-to-PDAC progression. However, immune interception strategies require full understanding of PanIN and PDAC cellular architecture. Surgical specimens containing PanIN and PDAC lesions from a unique cohort of 5 treatment-naive patients with PDAC were surveyed using spatial omics (proteomic and transcriptomic). Findings were corroborated by spatial proteomics of PanIN and PDAC from tamoxifen-inducible KPC mice. We uncovered the organization of lymphoid cells into tertiary lymphoid structures (TLSs) adjacent to PanIN lesions. These TLSs lacked CD21+CD23+ B cells compared with more mature TLSs near the PDAC border. PanINs harbored mostly CD4+ T cells, with fewer Tregs and exhausted T cells than PDAC. Peritumoral space was enriched with naive CD4+ and central memory T cells. These observations highlight the opportunity to modulate the immune microenvironment in PanINs before immune exclusion and immunosuppression emerge during progression into PDAC.
Authors
Melissa R. Lyman, Jacob T. Mitchell, Sidharth Raghavan, Luciane T. Kagohara, Amanda L. Huff, Saurav D. Haldar, Sarah M. Shin, Samantha Guinn, Benjamin Barrett, Gabriella Longway, Alexei Hernandez, Erin M. Coyne, Xuan Yuan, Lalitya Andaloori, Jiaying Lai, Yun Zhou Liu, Rachel Karchin, Anuj Gupta, Ashley L. Kiemen, André Forjaz, Denis Wirtz, Pei-Hsun Wu, Atul Deshpande, Jae W. Lee, Todd D. Armstrong, Nilofer S. Azad, Jacquelyn W. Zimmerman, Laura D. Wood, Robert A. Anders, Elizabeth D. Thompson, Elizabeth M. Jaffee, Elana J. Fertig, Won Jin Ho, Neeha Zaidi
Abstract
Alveolar epithelial type II (AT2) cell dysfunction is implicated in the pathogenesis of familial and sporadic idiopathic pulmonary fibrosis (IPF). We previously demonstrated that expression of an AT2 cell–exclusive disease-associated protein isoform (SP-CI73T) in murine and patient-specific induced pluripotent stem cell–derived (iPSC-derived) AT2 cells leads to a block in late macroautophagy and promotes time-dependent mitochondrial impairments; however, how a metabolically dysfunctional AT2 cell results in fibrosis remains elusive. Here, using murine and human iPSC-derived AT2 cell models expressing SP-CI73T, we characterize the molecular mechanisms governing alterations in AT2 cell metabolism that lead to increased glycolysis, decreased mitochondrial biogenesis, disrupted fatty acid oxidation, accumulation of impaired mitochondria, and diminished AT2 cell progenitor capacity manifesting as reduced AT2 cell self-renewal and accumulation of transitional epithelial cells. We identify deficient AMPK signaling as a critical component of AT2 cell dysfunction and demonstrate that targeting this druggable signaling hub can rescue the aberrant AT2 cell metabolic phenotype and mitigate lung fibrosis in vivo.
Authors
Luis R. Rodríguez, Konstantinos-Dionysios Alysandratos, Jeremy Katzen, Aditi Murthy, Willy Roque Barboza, Yaniv Tomer, Sarah Bui, Rebeca Acín-Pérez, Anton Petcherski, Kasey Minakin, Paige Carson, Swati Iyer, Katrina Chavez, Charlotte H. Cooper, Apoorva Babu, Aaron I. Weiner, Andrew E. Vaughan, Zoltan Arany, Orian S. Shirihai, Darrell N. Kotton, Michael F. Beers
Abstract
IL-33 is a key driver of type 2 inflammation and implicated in pathology of chronic obstructive pulmonary disease (COPD) and asthma. However, the mechanism for IL-33 secretion and regulation in the context of chronic airway disease is poorly understood. We previously reported an airway disease–associated isoform IL-33Δ34 that escapes nuclear sequestration and is tonically secreted from epithelial cells. Here, we describe how this IL-33Δ34 isoform interacts with HSP70 within cells and is targeted to secretory organelles through coordinated binding to phosphatidylserine (PS) and delivered to compartments for unconventional protein secretion (CUPS). Once secreted, extracellular HSP70 (eHSP70) in complex with IL-33Δ34 stabilizes the cytokine by inhibiting oxidation and degradation, which results in enhanced IL-33Δ34-receptor binding and activity. We further find evidence that IL-33 along with mediators of the proteostasis network HSP70, HSP90, and the Chaperonin Containing TCP1 (CCT) complex are dysregulated in human chronic airway disease. This phenomenon is reflected in the differential extracellular vesicle (EV) proteome in bronchial wash from COPD and asthma samples, which could mark disease activity and potentiate IL-33 function. This study confirms proteostasis intermediates, chiefly HSP70, as chaperones for noncanonical IL-33 secretion and activity that may be amenable for therapeutic targeting in the chronic airway diseases COPD and asthma.
Authors
Omar A. Osorio, Heather E. Raphael, Colin E. Kluender, Ghandi F. Hassan, Lucy S. Cohen, Deborah F. Steinberg, Ella Katz-Kiriakos, Morgan D. Payne, Ethan M. Luo, Jamie L. Hicks, Derek E. Byers, Jennifer Alexander-Brett
Abstract
High myopia (HM) and posterior staphyloma (PS) are major causes of vision loss worldwide. Genetic and environmental factors, especially light exposure, influence myopia. This study shows that low-density lipoprotein–related receptor type 2 (LRP2) levels are decreased in the vitreous of patients with HM and PS, and that in human donor eyes affected by PS, LRP2 expression was reduced in the neural retina and retinal pigment epithelium (RPE), with morphologic changes similar to those observed in the Foxg1-Cre-Lrp2fl/fl mouse that also develops PS. In human induced pluripotent stem cell–derived RPE cells, LRP2 silencing regulated genes involved in eye and neuronal development, visual perception, tissue remodeling, hormone metabolism, and RPE structure. Its expression increased under light exposure, particularly red light, but was downregulated by cortisol. These findings establish a link between LRP2, myopization, and environmental factors, highlighting its crucial role in nonsyndromic HM and PS. LRP2 appears to be a promising therapeutic target for HM treatment.
Authors
Kimberley Delaunay, Emilie Picard, Patricia Lassiaz, Laurent Jonet, Vidjea Cannaya, José Maria Ruiz-Moreno, Kentaro Kojima, Henrik Vorum, Bent Honoré, Jorge R. Medrano, Lasse Jørgensen Cehofski, Eric Pussard, Renata Kozyraki, Alicia Torriglia, Olivier Cases, Francine Behar-Cohen
Abstract
Mutations in Cullin-3 (CUL3) cause hypertension (HTN). We examined the role of smooth muscle cell (SMC) CUL3 in the regulation of renin gene expression. Mice with SMC-specific CUL3 deletion (S-CUL3-KO) developed severe HTN with paradoxically preserved levels of plasma angiotensin peptides and renal renin expression. Cre-recombinase was active in juxtaglomerular (JG) cells, resulting in decreased CUL3 expression. We evaluated components of the renin cell baroreceptor and revealed preserved Lamin A/C but decreased integrin β1 expression in S-CUL3-KO. We hypothesized that Rab proteins are involved in integrin β1 downregulation. Silencing either Rab21 or Rab5 in CUL3-deficient HEK293 cells increased integrin β1 protein. Coimmunoprecipitation revealed a direct interaction between Rab5 and CUL3. CUL3 deficiency increased Rab5, suggesting it is regulated by a CUL3-mediated mechanism and that CUL3 deficiency results in loss of Rab protein turnover, leading to enhanced integrin β1 internalization. We conclude that the loss of integrin β1 from JG cells impairs the mechanosensory function of the renin cell baroreceptor, which underlies the persistent renin expression observed in hypertensive S-CUL3-KO mice. These findings provide insights into the molecular mechanisms of HTN, revealing that dysregulation of Rab proteins and integrin β1 in the kidney due to CUL3 deficiency contributes to the development of HTN.
Authors
Daria Golosova, Gaurav Kumar, Ko-Ting Lu, Patricia C. Muskus Veitia, Ana Hantke Guixa, Kelsey K. Wackman, Eva M. Fekete, Daniel T. Brozoski, Justin L. Grobe, Maria Luisa S. Sequeira-Lopez, R. Ariel Gomez, Pablo Nakagawa, Curt D. Sigmund
Abstract
Extracellular DNA (ecDNA) released from injured and dying cells powerfully induces injurious inflammation. In this study we define the role of ecDNA in systemic vasculitis affecting the kidney, using human kidney biopsies and murine models of myeloperoxidase anti-neutrophil cytoplasmic antibody-associated glomerulonephritis (MPO-ANCA GN). Twice daily administration of intravenous deoxyribonuclease I (ivDNase I) in 2 models of anti-MPO GN reduced glomerular deposition of ecDNA, histological injury, leukocyte infiltration, and NETosis. Comprehensive investigation into DNase I modes of action revealed that after exposure to MPO, DNase I reduced lymph node DC numbers and their activation status, resulting in decreased frequency of MPO-specific CD4+ effector T cells (IFN-γ and IL-17A producing) and reductions in dermal anti-MPO delayed type hypersensitivity responses. To overcome the translational obstacle of the short half-life of DNase I (<5 hours), we tested an adeno-associated viral vector encoding DNase I. This method of DNase I delivery was more effective, as in addition to the histological and antiinflammatory changes described above, a single vector treatment also reduced circulating MPO-ANCA titers and albuminuria. These results indicate ecDNA is a potent driver of anti-MPO GN and DNase I is a potential therapeutic that can be delivered using gene technology.
Authors
Anne Cao Le, Virginie Oudin, Jonathan Dick, Maliha A. Alikhan, Timothy A. Gottschalk, Lu Lu, Kate E. Lawlor, Daniel Koo Yuk Cheong, Mawj Mandwie, Ian E. Alexander, A.R. Kitching, Poh-Yi Gan, Grant J. Logan, Kim M. O’Sullivan
Abstract
The Z variant (Glu342Lys) causes alpha-1 antitrypsin (AAT) to self-assemble into polymer chains that accumulate within hepatocytes, causing liver disease and exposing a cryptic epitope recognized by the 2C1 monoclonal antibody (mAb). They can be blocked by the small molecule GSK716 (‘716) that stabilizes an intermediate on the polymerization pathway. We have characterized 23 mutants of AAT in a cellular model to establish: (a) their ability to form intracellular polymers, (b) whether polymer formation could be prevented by ‘716, and (c) whether the polymers expose the 2C1 cryptic epitope. Most of the variants, including Mprocida (Leu41Pro), Mherleen (Pro369Leu), Mduarte (Asp256Val), Lfrankfurt (Pro255Thr), Yorzinuovi (Pro391His), Mwurzburg (Pro369Ser), and p.289S accumulated as intracellular polymers. Eleven formed polymers that were resistant to ‘716, including Mprocida, Mmalton (ΔPhe51), Lfrankfurt, Mduarte, S (Glu264Val), Mherleen, and Yorzinuovi. The ‘716 resistant mutants localize to a region of the AAT molecule separate from the binding site of the small molecule and form polymers that are less well recognized by the 2C1 mAb. They are fully recognized by a novel 8A7 mAb that we developed to have a broader specificity. Our data suggest that individuals with these mutations are unlikely to benefit from treatment with ‘716 or its derivatives.
Authors
Riccardo Ronzoni, Ibrahim Aldobiyan, Elena Miranda, Narinder Heyer-Chauhan, Emma L.K. Elliston, Juan Pérez, Annamaria Fra, James A. Irving, David A. Lomas
No posts were found with this tag.