Research ArticleNeuroscienceTherapeutics
Open Access |  10.1172/jci.insight.183189
10.1172/jci.insight.183189
Systemic gene therapy corrects the neurological phenotype in a mouse model of NGLY1 deficiency
Ailing Du,1 Kun Yang,2,3 Xuntao Zhou,1 Lingzhi Ren,1 Nan Liu,1 Chen Zhou,1 Jialing Liang,1 Nan Yan,2,3 Guangping Gao,1,4 and Dan Wang1,5
1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by Du, A. in: PubMed | Google Scholar
1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by
Yang, K.
in:
PubMed
|
Google Scholar
|

1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by Zhou, X. in: PubMed | Google Scholar
1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by Ren, L. in: PubMed | Google Scholar
1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by Liu, N. in: PubMed | Google Scholar
1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by Zhou, C. in: PubMed | Google Scholar
1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by Liang, J. in: PubMed | Google Scholar
1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by Yan, N. in: PubMed | Google Scholar
1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by
Gao, G.
in:
PubMed
|
Google Scholar
|

1Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
2Department of Immunology and
3Department of Microbiology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
4Department of Microbiology and Physiological Systems and
5RNA Therapeutics Institute, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
Find articles by
Wang, D.
in:
PubMed
|
Google Scholar
|

Published August 13, 2024 - More info
JCI Insight. 2024;9(19):e183189. https://doi.org/10.1172/jci.insight.183189.
© 2024 Du et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Received: May 20, 2024; Accepted: August 7, 2024
-
Abstract
The cytoplasmic peptide:N-glycanase (NGLY1) is ubiquitously expressed and functions as a de–N-glycosylating enzyme that degrades misfolded N-glycosylated proteins. NGLY1 deficiency due to biallelic loss-of-function NGLY1 variants is an ultrarare autosomal recessive deglycosylation disorder with multisystemic involvement; the neurological manifestations represent the main disease burden. Currently, there is no treatment for this disease. To develop a gene therapy, we first characterized a tamoxifen-inducible Ngly1-knockout (iNgly1) C57BL/6J mouse model, which exhibited symptoms recapitulating human disease, including elevation of the biomarker GlcNAc-Asn, motor deficits, kyphosis, Purkinje cell loss, and gait abnormalities. We packaged a codon-optimized human NGLY1 transgene cassette into 2 adeno-associated virus (AAV) capsids, AAV9 and AAV.PHPeB. Systemic administration of the AAV.PHPeB vector to symptomatic iNgly1 mice corrected multiple disease features at 8 weeks after treatment. Furthermore, another cohort of AAV.PHPeB-treated iNgly1 mice were monitored over a year and showed near-complete normalization of the neurological aspects of the disease phenotype, demonstrating the durability of gene therapy. Our data suggested that brain-directed NGLY1 gene replacement via systemic delivery is a promising therapeutic strategy for NGLY1 deficiency. Although the superior CNS tropism of AAV.PHPeB vector does not translate to primates, emerging AAV capsids with enhanced primate CNS tropism will enable future translational studies.
-
Introduction
N-glycanase 1 (NGLY1) deficiency (OMIM #615273) is an ultrarare autosomal recessive deglycosylation disorder with multisystemic involvement, including global developmental delay, movement disorder, a reduction or lack of tears, transient elevation of liver enzymes, and scoliosis (1–9). More than 100 patients have been reported worldwide. It is caused by biallelic loss-of-function variants in the NGLY1 gene (10–13) that encodes peptide:N-glycanase (PNGase, aka N-glycanase 1) (14–16). NGLY1 plays an integral role in the endoplasmic reticulum–associated degradation pathway by clearing misfolded glycoproteins. It cleaves the aspartyl glycosylamine bond of N-linked glycoproteins to produce a free oligosaccharide and a deaminated protein, which are further degraded in the lysosome and by the proteasome, respectively (17–19) (Figure 1A). Under NGLY1 deficiency, another deglycosylating enzyme known as ENGase removes N-glycans by cleaving the glycosidic bond between the 2 GlcNAc residues, leaving a single GlcNAc residue attached to the protein. This incompletely deglycosylated protein is prone to aggregation (17, 20), contributing to dysregulation of several cellular processes and pathogenesis. Furthermore, degradation of GlcNAc-protein generates GNA that is inversely correlated to the NGLY1 function (17, 21) (Figure 1A). GNA levels are consistently elevated in NGLY1-deficient cells, animal models, and patients and can serve as a biomarker for NGLY1 deficiency (22–24).
 Figure 1
Figure 1NGLY1 knockdown in an inducible knockout mouse model of NGLY1 deficiency. (A) Schematic cartoon showing the biological function of NGLY1 and the generation of N-GlcNAc (GNA) in its absence. ENGase, endo-β-N-acetylglucosaminidase. (B) A part of the genome structure of mouse loxP-flanked (flox or fl) Ngly1fl allele. The endogenous Ngly1 locus carries 2 loxP sites flanking exons 11 and 12. (C) Timeline of tamoxifen administration and workflow to induce Ngly1 conditional knockout in mice. (D) Quantification of endogenous mouse Ngly1 mRNA expression (cDNA) in the brain, spinal cord, liver, heart, and tibialis anterior (TA) muscle of Ngly1fl/fl and Ngly1fl/fl iCre mice. All mice were treated with tamoxifen as shown in C and euthanized on postnatal day 56 (P56). N = 8 mice (4 males and 4 females) per group. (E) Representative Western blotting images of NGLY1 protein expression in the brain, spinal cord, liver, heart, and TA muscle of the mice as shown in D (males only). Quantification of NGLY1 signal (normalized to GAPDH expression) is shown below the images. N = 4 mice per group. (F) GNA levels in various tissues of Ngly1fl/fl mice and Ngly1fl/fl iCre mice. N = 6 mice (3 males and 3 females) per group. In D–F data are mean ± SD of biological repeats; each white dot represents an individual mouse (circle: female; square: male). Statistical analysis was performed by 2-tailed Student’s t test.
The pathogenesis of NGLY1 deficiency is still poorly understood, and no effective therapy is currently available. Intriguingly, ENGase gene deletion can reverse abnormal GlcNAc-protein accumulation in Ngly1-knockout mouse embryonic fibroblasts (25) and partially rescue the lethality of NGLY1-deficient mice (26). Several small molecule ENGase inhibitors were discovered in the FDA-approved drug database and considered as potential treatments for NGLY1 deficiency (27). Besides clearing misfolded proteins, recent reports showed that the deglycosylation function of NGLY1 also regulates biologically relevant proteins, such as nuclear factor erythroid-2-like 1 (28–32) and Na+-K+-Cl− co-transporter 1 (33, 34), and cellular pathways, such as BMP signaling (35, 36) and AMPK signaling (37, 38). In addition, the nonenzymatic, regulatory function of NGLY1 in aquaporin transcription has been reported (39). The pathophysiological basis of NGLY1 deficiency may be associated with the absence of various enzymatic and nonenzymatic roles of NGLY1 (40). Therefore, gene replacement therapy may achieve maximal NGLY1 function restoration by addressing the root cause of NGLY1 deficiency.
In a previous study, Asahina et al. generated recombinant adeno-associated virus serotype 9 (rAAV9) vector expressing human NGLY1 cDNA and tested its therapeutic efficacy in an Ngly1-knockout (Ngly1–/–) rat model. A single intracerebroventricular (ICV) administration at the dose of 2 × 1010 vector genomes (vg)/rat restored NGLY1 expression in the brain and spinal cord and normalized the motor phenotype of Ngly1–/– rats (41, 42). More recently, Zhu et al. compared 3 routes of administration of an AAV9-based gene therapy vector (GS-100) in the same rat model, namely intravenous (IV), ICV, and IV+ICV, and found that ICV and IV+ICV administration resulted in widespread transgene delivery and expression throughout the CNS, concomitant with significant reduction in GNA in the CNS and behavioral improvements compared with untreated Ngly1–/– rats. IV-only administration did not provide any benefit, and IV+ICV did not provide additional benefit compared with ICV only (43). Together, these studies suggest that CNS-directed gene therapy is a promising treatment strategy for NGLY1 deficiency.
Although ICV administration of rAAV9 leads to widespread transgene delivery throughout rodent brains, achieving the same targeting efficiency in the human brain can be challenging because of its much larger size (44). Unlike certain lysosomal enzymes that can be secreted and taken up by other cells, the cytosolic NGLY1 protein functions in a cell-autonomous manner. Therefore, NGLY1 gene therapy only rescues transduced cells, and a broader coverage of CNS gene delivery likely correlates with a better therapeutic outcome. Consistent with this notion, ICV delivery of GS-100 at a very high dose of 6 × 1012 vg/rat only resulted in 50% or less GNA reduction in CNS tissues of Ngly1–/– rats, presumably limited by the number of transduced cells (43). Systemic delivery of certain rAAVs that can cross the blood-brain barrier (BBB) has the potential to broadly transduce the CNS owing to its high density of capillaries. However, IV delivery of GS-100 at a high dose of 1 × 1014 vg/kg to Ngly1–/– rats did not result in sufficient CNS transduction to confer any therapeutic benefit (43). Recently, several groups engineered AAV capsids to cross the BBB more efficiently than AAV9 in mice (45–47) or nonhuman primates (NHPs) (48–51), potentially enabling noninvasive systemic gene therapy development for myriad neurological disorders afflicting broad CNS regions, such as NGLY1 deficiency.
In this study, we set out to test whether systemic delivery of a potent BBB-crossing rAAV can lead to therapeutic outcome in an NGLY1 deficiency animal model. To this end, we turned to the well-characterized AAV.PHPeB capsid that exhibits 50- to 100-fold higher CNS transduction than AAV9 following systemic injection in C57BL/6 mice (46). Because the superior BBB-crossing property of AAV.PHPeB does not translate to rats (52–54), and Ngly1–/– mice on C57BL/6 background are embryonically lethal (26), we first generated an inducible Ngly1-knockout C57BL/6 mouse model (iNgly1). In the iNgly1 mouse model, the last 2 exons of Ngly1 are flanked by a pair of loxP sites (Ngly1fl/fl) (17, 30, 55) (Figure 1B), and it carries a tamoxifen-inducible, ubiquitously expressed Cre transgene (iCre). Therefore, tamoxifen treatment leads to whole-body Ngly1 knockout. We found that iNgly1 mice exhibited a range of disease-related phenotypes following tamoxifen treatment, including movement disorder, neurological impairment, scoliosis, and reduced fitness and survival. Systemic injection of an AAV.PHPeB gene replacement vector after disease onset significantly ameliorated the disease phenotype up to 14 months of age. Our data suggested that CNS-directed NGLY1 gene replacement therapy via systemic delivery is a promising therapeutic strategy for NGLY1 deficiency.
-
Results
Ngly1 knockdown in iNgly1 mice led to a disease-related phenotype. To induce Ngly1 knockout, iNgly1 mice received 2 rounds of tamoxifen treatment during postnatal day 7 (P7) to P9 and P31 to P33, respectively; their phenotype was assessed on P56 (Figure 1C). Quantification of Ngly1 mRNA and NGLY1 protein in multiple tissues demonstrated efficient gene expression knockdown by 80%–90% in the iNgly1 mice compared with the Ngly1fl/fl littermates (i.e., without the iCre transgene and treated with tamoxifen in the same manner) (Figure 1, D and E). Correspondingly, elevated GNA levels were observed in all tissues examined, including brain, spinal cord, liver, heart, and TA muscle (Figure 1F). Compared with Ngly1fl/fl controls, the iNgly1 mice showed lower body weight, reduced number of Calbindin+ Purkinje cells in the cerebellum, and motor function abnormalities as revealed by rotarod test and CatWalk gait analysis (Figure 2, A–D). They also exhibited severe kyphosis as revealed by x-ray radiography (Figure 2E). Together, these results demonstrated that the iNgly1 mouse model recapitulated the key clinical features of NGLY1 deficiency in human patients, such as motor impairment and scoliosis.
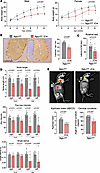 Figure 2
Figure 2NGLY1 knockdown in iNgly1 mice leads to disease-related phenotype. (A) Body weight of Ngly1fl/fl and Ngly1fl/fl iCre mice as shown in Figure 1C. Data are reported as the mean ± SD of 4–9 mice per group. (B) Representative immunohistochemistry (IHC) images of Calbindin, a cellular marker for Purkinje cells, in the cerebellum of Ngly1fl/fl and Ngly1fl/fl iCre mice. Quantification of Calbindin+ Purkinje cells is shown to the right of the images. N = 8 mice (4 males and 4 females) per group. Scale bar: 200 μm. (C) Rotarod test for Ngly1fl/fl and Ngly1fl/fl iCre mice. N = 8–12 mice (4 to 5 males and 4 to 7 females) per group. (D) Quantification of stride length, paw max intensity, and paw single stance in the CatWalk gait analysis for Ngly1fl/fl and Ngly1fl/fl iCre mice. N = 8 to 13 mice (4 to 7 males and 4 to 6 females) per group. (E) Representative x-ray images showing kyphosis (yellow arrows). Quantification of kyphosis index (KI; the ratio of AB to CD) and cervical curvature angle is shown below the images. N = 8 to 13 mice (4 to 7 males and 4 to 6 females) per group. In A–E, data are mean ± SD of biological repeats; each white dot represents an individual mouse (circle: female; square: male). In A, statistical analysis was performed by 2-way ANOVA, followed by Tukey’s multiple comparisons test. In B–E, statistical analysis was performed by 2-tailed Student’s t test.
Short-term assessment on therapeutic efficacy of systemic gene therapy. We designed a gene replacement vector construct that expresses codon-optimized human NGLY1 cDNA driven by a ubiquitous promoter (opt-hNGLY1) (Figure 3A) and packaged it in AAV9 and PHPeB, an engineered capsid showing more efficient CNS transduction than AAV9 by IV injection in C57BL/6 mice (46). Following the same tamoxifen treatment regimen to induce endogenous Ngly1 knockout, rAAV was injected to mice via tail vein at 1.5 × 1012 vg/mouse on P56 (Figure 3B), when iNgly1 mice showed a disease-related phenotype (Figure 2). Eight weeks later (i.e., on P112), mice were subjected to molecular analysis and phenotype assessment. Consistent with the phenotype characterization study (Figure 1D), the endogenous Ngly1 mRNA in the iNgly1 brain was reduced by 80%–90% regardless of rAAV treatment (Figure 3C). As expected, rAAV.PHPeB treatment led to approximately 40-fold higher vector genome copy numbers and opt-hNGLY1 expression in the brain than rAAV9 (Figure 3, D and E).
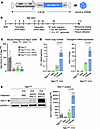 Figure 3
Figure 3Comparing rAAV9 and rAAV.PHPeB for gene delivery and expression efficiency. (A) Schematic diagram showing the vector constructs expressing the codon-optimized human NGLY1 gene (opt-hNGLY1). (B) Timeline of tamoxifen administration and workflow to induce Ngly1 knockout in mice followed by rAAV treatment and phenotype assessment. (C) Quantification of mouse endogenous Ngly1 mRNA expression (cDNA) in the brain. N = 4–6 mice per group. (D) Quantification of vector genome copy and hNGLY1 transcript (reverse-transcribed into cDNA) in the brain. (E) Representative Western blotting images of NGLY1 protein expression in the brain of the mice as shown in C. Quantification of NGLY1 signal (normalized to GAPDH expression) is shown. N = 5 to 8 mice per group. In C–E, data are mean ± SD of biological repeats; each white dot represents an individual mouse (circle: female; square: male). Statistical analysis was performed by 1-way ANOVA followed by Tukey’s multiple comparisons test.
Although rAAV9 treatment did not improve body weight of iNgly1 mice, rAAV.PHPeB treatment resulted in marginal body weight gain in males (Figure 4A). The superior therapeutic efficacy of rAAV.PHPeB was evidenced by restoration of Calbindin+ Purkinje cells in the cerebellum and normalized performance on rotarod (Figure 4, B and C). In the CatWalk gait analysis, mice receiving rAAV.PHPeB treatment showed equal or better correction compared with rAAV9, though the trend did not reach statistical significance after correcting for multiple comparisons (Figure 4D). Kyphosis was largely rescued by rAAV.PHPeB treatment but not by rAAV9 (Figure 5, A and B). These results led us to conclude that CNS gene delivery was of utmost importance to ameliorate the disease phenotype in iNgly1 mice. We therefore chose rAAV.PHPeB in the following long-term study.
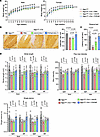 Figure 4
Figure 4Comparing rAAV9 and rAAV.PHPeB for short-term therapeutic efficacy. (A) Body weight of mice as shown in Figure 3B. Data are reported as the mean ± SD of 2–10 mice per group. (B) Representative immunohistochemistry (IHC) images of Calbindin in the cerebellum of Ngly1fl/fl and Ngly1fl/fl iCre mice with or without rAAV treatment. Quantification of Calbindin+ Purkinje cells is shown on the right. N = 3 to 4 male mice per group. Scale bar: 200 μm. (C) Rotarod test when mice were at the age of 16 weeks. N = 5–17 mice per group. (D) Quantification of stride length, paw max intensity, and paw single stance in the CatWalk gait analysis. N = 5 to 17 mice per group. In A, data are reported as the mean ± SD. Statistical analysis was performed by 2-way ANOVA, followed by Tukey’s multiple comparisons test. In B–D, data are mean ± SD of biological repeats; each white dot represents an individual mouse (circle: female; square: male). Statistical analysis was performed by 1-way ANOVA followed by Tukey’s multiple comparisons test.
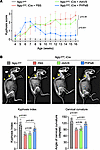 Figure 5
Figure 5rAAV.PHPeB outperforms rAAV9 in correcting kyphosis. (A) Kyphosis scores of Ngly1fl/fl mice and Ngly1fl/fl iCre mice with or without rAAV treatment. N = 5–17 mice per group. (B) Representative x-ray images showing kyphosis (yellow arrows). Quantification of kyphosis index (KI) and cervical curvature angle is shown below the images. N = 5 to 17 mice per group. In A, data are reported as the mean ± SD. Statistical analysis was performed by 2-way ANOVA, followed by Tukey’s multiple comparisons test. In B, data are mean ± SD of biological repeats; each white dot represents an individual mouse (circle: female; square: male). Statistical analysis was performed by 1-way ANOVA followed by Tukey’s multiple comparisons test.
rAAV.PHPeB treatment showed long-term therapeutic efficacy in iNgly1 mice. To examine whether a single dose of rAAV.PHPeB treatment on P56 could afford durable therapeutic efficacy, another cohort of mice were monitored until 14 months of age, when over half of untreated iNgly1 mice succumbed to the disease (Figure 6A). rAAV.PHPeB treatment rescued the lethality and modestly but significantly improved body weight at 14 months of age (Figure 6, A and B). Calbindin+ Purkinje cells and motor function were largely preserved in iNgly1 mice receiving rAAV.PHPeB treatment (Figure 6, C–E). Kyphosis was significantly corrected and stabilized in rAAV.PHPeB-treated iNgly1 mice when assessed until 14 months of age (Figure 7).
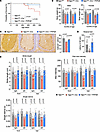 Figure 6
Figure 6rAAV.PHPeB treatment shows long-term therapeutic efficacy in iNgly1 mice. (A) Kaplan-Meier plot of the survival rate of Ngly1fl/fl mice and Ngly1fl/fl iCre mice with or without rAAV treatment. All mice received tamoxifen treatment as indicated in Figure 3B. N ≥ 18 mice per group with similar female/male ratios in each group. (B) Body weight at the age of 11 and 14 months. N ≥ 6 mice per group. (C) Representative immunohistochemistry (IHC) images of Calbindin, a cellular marker for Purkinje cells, in the cerebellum. Quantification of Calbindin+ Purkinje cells is shown on the right. N = 3 to 5 male mice per group. Scale bar: 200 μm. (D) Rotarod test when mice were at the age of 14 months. N = 12 to 21 mice per group. (E) Quantification of stride length, paw max intensity, and paw single stance in the CatWalk gait analysis. N = 12 to 24 mice per group. In A, statistical analysis was performed by log-rank (Mantel-Cox) test. In B–E, data are mean ± SD of biological repeats; each white dot represents an individual mouse (circle: female; square: male). Statistical analysis was performed by 1-way ANOVA followed by Tukey’s multiple comparisons test.
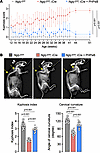 Figure 7
Figure 7rAAV.PHPeB treatment rescues kyphosis in iNgly1 mice. (A) Kyphosis scores of Ngly1fl/fl mice and Ngly1fl/fl iCre mice with or without rAAV.PHPeB treatment over time. N ≥ 12 mice per group with similar female/male ratios in each group. (B) Representative x-ray images showing kyphosis (yellow arrows). Quantification of kyphosis index (KI) and cervical curvature angle is shown below the images. N = 12 to 25 mice per group. In A, data are reported as the mean ± SD. Statistical analysis was performed by 2-way ANOVA, followed by Tukey’s multiple comparisons test. In B, data are mean ± SD of biological repeats; each white dot represents an individual mouse (circle: female; square: male). Statistical analysis was performed by 1-way ANOVA followed by Tukey’s multiple comparisons test.
At the molecular level, we verified that endogenous Ngly1 mRNA knockdown was comparable between the iNgly1 mice with or without rAAV.PHPeB treatment (Figure 8A), ruling out inefficient gene knockout as a reason for phenotype difference between the 2 groups. Surprisingly, we did not observe GNA reduction in peripheral tissues, including liver, heart, and TA muscle (Figure 8B), despite NGLY1 protein restoration at levels near or above normal (Figure 8C). However, we cannot rule out the possibility that the anti-NGLY1 antibody used in Western blotting may recognize the human NGLY1 protein more efficiently than mouse NGLY1 protein. Although rAAV.PHPeB treatment significantly reduced GNA levels in the spinal cord and most iNgly1 mouse brains, it failed to rescue elevated brain GNA levels in 2 iNgly1 mice (Figure 8B). Western blotting using tissue lysates revealed that these 2 brain samples contained much less NGLY1 protein than the other 3 samples in the same group, likely due to technical variations leading to inefficient gene delivery to the brain (Figure 8C). Therefore, the inverse correlation between GNA levels and NGLY1 protein abundance in the brain (Figure 8D) indicates that efficient gene delivery is required to effectively reduce GNA in the brain.
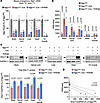 Figure 8
Figure 8rAAV.PHPeB treatment lowers GNA in the CNS of iNgly1 mice. (A) Quantification of mouse endogenous Ngly1 mRNA expression (cDNA) in the brain, spinal cord, liver, heart, and TA muscle. N = 5 to 6 mice per group. (B) GNA levels in various tissues of Ngly1fl/fl mice and Ngly1fl/fl iCre mice with or without rAAV.PHPeB treatment. N = 5 mice per group. (C) Representative Western blotting images of NGLY1 protein expression in the brain, spinal cord, liver, heart, and TA muscle of the mice. Quantification of NGLY1 signal (normalized to GAPDH expression) is shown below the images. N = 5 to 6 mice per group. (D) Correlation between GNA level (y axis) and NGLY1 protein expression (x axis) in the brain of Ngly1fl/fl iCre mice with or without rAAV.PHPeB treatment. Each dot represents 1 animal. In A–C, data are mean ± SD of biological repeats; each white square represents an individual male mouse. In A and B, statistical analysis was performed by 1-way ANOVA followed by Tukey’s multiple comparisons test. In C, statistical analysis was performed by 2-tailed Student’s t test. In D, statistical analysis was performed by simple linear regression.
-
Discussion
The groundbreaking discovery that a noninvasive systemic administration of rAAV9 can cross the BBB and achieve widespread CNS gene delivery (56, 57) has opened an exciting avenue to address neurological diseases, especially the conditions afflicting broad CNS compartments. CNS-targeted IV rAAV gene therapy has been employed in several clinical applications, exemplified by onasemnogene abeparvovec (aka Zolgensma), an FDA-approved treatment for spinal muscular atrophy (SMA) (58). In postmortem tissues from 2 patients with SMA receiving Zolgensma at 1.1 × 1014 vg/kg, transgene biodistribution was evident throughout the CNS, reaching 0.1–1 vg/diploid genome in most brain and spinal cord samples (59). Despite the encouraging preclinical and clinical results of IV rAAV9 for SMA and other neurological diseases (60), a recent study failed to demonstrate the effectiveness of IV rAAV9 gene replacement therapy (1 × 1014 vg/kg) in an Ngly1–/– Sprague-Dawley rat model (43). It was likely attributable to the poor CNS biodistribution of vector DNA that was below 0.01 vg/diploid genome in most brain regions. In contrast, ICV administration resulted in therapeutic efficacy in the same Ngly1–/– rat model, which was correlated with approximately 10-fold higher vector DNA biodistribution in the CNS (43). Therefore, we hypothesized that IV administration of a more potent BBB-crossing capsid vector can lead to meaningful therapeutic efficacy for NGLY1 deficiency and have translational value.
The unrealized potential of IV rAAV gene therapy for a broad range of neurological diseases has led to considerable efforts to engineer AAV capsid for higher BBB-crossing efficiency in multiple animal species. Notably, most resulting AAV capsid variants exhibit a species-specific property, i.e., they retain enhanced performance only in the animal species that is originally used to identify them. For example, PHP.B and PHPeB capsids were shown to significantly outperform AAV9 in C57BL/6 mice (45, 46, 61). Although the superb BBB-crossing property of neither capsid translates to NHPs (62, 63), both capsids provide powerful tools to investigate the feasibility of CNS-targeted IV rAAV gene therapy for neurological diseases, such as Rett syndrome (64), fragile X syndrome (65), Dravet syndrome (66), Niemann-Pick disease (67), Pompe disease (68), and synucleinopathy (69). Recently, exciting progress has been made to engineer AAV capsid variants that can drastically outperform AAV9 in crossing the primate BBB (48–51), paving the way for future clinical development of CNS-targeted IV rAAV gene therapy. However, these primate-specific capsids do not perform equally well in mice (48) and therefore are not suitable for preclinical testing in disease mouse models.
Utilizing the PHPeB vector for NGLY1 deficiency preclinical gene therapy study faces 2 major challenges. First, Ngly1–/– mice on C57BL/6 background are embryonically lethal (26), precluding rAAV administration in postnatal animals. Second, the superb CNS-crossing property of PHPeB does not translate to rats, so the Ngly1–/– rat model is unlikely to respond. To overcome these challenges, we first generated the iNgly1 mouse model on a C57BL/6 background that showed 80%–90% reduction in Ngly1 expression, concomitant with disease phenotype that recapitulated human NGLY1 deficiency, including elevated GNA, failure to thrive, loss of Purkinje cells, motor impairment, and kyphosis. This mouse model allowed us to leverage the PHPeB capsid to study whether enhanced CNS penetration of IV rAAV gene therapy can be efficacious for NGLY1 deficiency and to compare with the gold-standard AAV9 capsid. As expected, PHPeB vector substantially outperformed rAAV9 for CNS gene transfer following IV administration in symptomatic iNgly1 mice and resulted in near-complete phenotypic correction when examined at 8 weeks after injection. The PHPeB-mediated therapeutic efficacy was also evident at 14 months postinjection, supporting the durability of gene replacement therapy for NGLY1 deficiency.
Clinical translation of IV rAAV gene therapy for neurological diseases such as NGLY1 deficiency is not without challenges. The preexisting anti-AAV capsid neutralizing antibody (NAb) in the general human population can compromise or abolish gene transfer via the bloodstream. To diminish circulating NAb titers to a level permissive to rAAV administration, a pretreatment of immunoglobin-cleaving enzymes has shown promising results in mouse and NHP studies (70, 71). Another major concern is the requirement of high doses of IV rAAV to enable BBB penetration, which has been associated with toxicities in clinical settings (72) and high vector manufacturing cost. Recently, several engineered AAV capsids were identified to efficiently cross NHP (48–51) and humanized (73) BBB. These emerging capsids can potentially afford adequate pan-CNS gene transfer with lower doses and therefore improve the safety and practicality of CNS-targeted IV rAAV gene therapy.
In summary, our study reinforces the feasibility of rAAV gene replacement therapy for NGLY1 deficiency and suggests that IV delivery can lead to therapeutic efficacy provided adequate CNS penetration using a suitable capsid vector.
-
Methods
Sex as a biological variable
We included both sexes in most experiments and noted males and females as squares and circles, respectively. In the Calbindin immunohistochemistry experiments, we assessed only male mice as a representative sex, but we expected the results to be relevant to female mice as well.
Animal use and treatment
The Ngly1fl/fl iCre mouse line was generated by crossing a mouse line harboring loxP-flanked (floxed) Ngly1 (a gift from Tadashi Suzuki, RIKEN Global Research Cluster, Wako, Saitama, Japan) and the UBC-Cre-ERT2 driver line (The Jackson Laboratory, strain: 007001) and is homozygous for the floxed Ngly1 allele (Ngly1fl/fl) and hemizygous for the inducible UBC-Cre-ERT2 transgene (iCre). The day of birth was designated P0. Ngly1fl/fl iCre mice were genotyped by PCR using primers Cre-F: 5′-GACGTCACCCGTTCTGTTG-3′ and Cre-R: 5′-AGGCAAATTTTGGTGTACGG-3′, which generates a 475 bp PCR amplicon indicating the presence of the iCre transgene. Tamoxifen (MilliporeSigma, T5648) was dissolved in corn oil (MilliporeSigma, C8267) to a concentration of 2 mg/mL (for P7–P9 pups) or 20 mg/mL (for P31–P33 weanlings), then administered to mice via intraperitoneal injection at a dose of 75 mg/kg. AAV vectors were diluted in Dulbecco’s PBS (MilliporeSigma, D8537-6X500ML), and 300 μL of diluted AAV vector was injected into mice via tail vein at a dose of 1.5 × 1012 vg/mouse. The number of animals used in each assay is detailed in the Supporting Data Values file. We included a larger cohort of animals for behavioral assays because of the inherent high variability associated with those assays and randomly selected a sex-balanced subset of animals for molecular analyses (e.g., GNA, DNA, RNA, protein quantification) to lower the cost. All data from the assessed animals are reported in the Supporting Data Values file. The body weight measurement was a combination of cross-sectional and longitudinal studies, resulting in some animals not being assessed at all time points.
Western blotting. Mouse tissues were homogenized using TissueLyser II (QIAGEN) in ice-cold T-PER (Thermo Fisher Scientific, 78510) with protease inhibitor (Roche, 4693159001). Total protein concentration was determined by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, 23225). Protein lysates normalized for total protein amount were boiled with 4× Laemmli sample buffer (Bio-Rad, 1610747) at 99°C for 5 minutes. Primary antibodies were rabbit anti-NGLY1 (MilliporeSigma, HPA036825, 1:1,000) and mouse anti-GAPDH (Abcam, ab8245, 1:10,000). Secondary antibodies were LI-COR IRDye 680RD goat anti-mouse IgG (H + L) (LI-COR Biosciences, 926-68070, 1:7,000) and LI-COR IRDye 800CW goat anti-rabbit IgG (H + L) (LI-COR Biosciences, 926-32211, 1:7,000). Blot membranes were imaged by LI-COR scanner (Odyssey) and quantified by LI-COR software.
Immunohistochemistry
Mice were perfused intracardially with ice-cold PBS, and tissues were dissected and immersion-fixed in 4% paraformaldehyde (Electron Microscopy Sciences, 15710) for 16–24 hours at 4°C followed by embedding in paraffin. Sectioning and immunohistochemistry with rabbit anti-Calbindin antibody (Abcam, ab229915, 1:3,000) were performed by the Morphology Core of University of Massachusetts Chan Medical School. Slides were imaged with a Leica Thunder Microscope. Quantification of the Calbindin+ cell number was performed using ImageJ Fiji. Three to 4 sagittal sections from a brain hemisphere were analyzed for each mouse as previously described (74). The multi-point tool in ImageJ was used to mark the number of Calbindin+ cells; the length of the Purkinje cell layer was measured with the segmented line tool.
X-ray radiography and kyphosis assessment
Radiographs of anesthetized mice in lateral position were obtained using an x-ray chamber (Trident digital x-ray machine, Hologic) at the Bone Core of University of Massachusetts Chan Medical School as previously reported (75). Quantification of kyphosis index was performed as previously described (75, 76). Briefly, a line AB was drawn from the posterior edge of the seventh segment of cervical vertebra (C7) to the posterior edge of the sixth lumbar spine (L6); another line CD was from the dorsal border of the vertebral body farthest from and perpendicular to the line AB. The lengths of AB and CD were measured using ImageJ Fiji; kyphosis index was defined as the ratio AB/CD.
Kyphosis score
Kyphosis severity was scored as previously described (77). Briefly, each mouse is observed on a flat surface. If the mouse can easily straighten its spine when it walks and does not have persistent kyphosis, it is scored 0. If the mouse has mild kyphosis but can straighten its spine, it is scored 1. If the mouse cannot straighten its spine completely and exhibits persistent but mild kyphosis, it is scored 2. If the mouse maintains pronounced kyphosis while it walks or rests, it is scored 3.
Behavioral tests
All behavioral tests were performed at the Mouse Behavioral Core Facility of University of Massachusetts Chan Medical School. Mice were maintained under a 12-hour light/12-hour dark cycle, and all behavioral tests were performed during the light phase. Mice were acclimated to the test room environment for 30 minutes before testing. Unless otherwise stated, all equipment was cleaned with 70% ethanol between trials to provide a standardized testing environment.
Rotarod test. Each mouse underwent 2 days of training sessions and 1 day of test sessions. Each session consisted of 3 separate 4-minute trials with a 15-minute interval between trials. The rotarod’s speed was accelerated from 4 to 40 rpm in 4 minutes at an accelerating rate of 0.15 revolutions per second. The time that each animal stayed on the rolling rod without falling was recorded. Each mouse was subjected to 3 tests, and the best performance was recorded. If a mouse remained on the rod at the end of the 4-minute trial, a time of 240 seconds was recorded.
CatWalk gait analysis. Gait analysis was performed using the CatWalk XT system (Noldus) (78). Mice were trained on an enclosed glass platform at least 3 times before gait analysis began. Each mouse was placed on the open end of the glass plate and allowed to walk across the entire glass floor voluntarily. A high-speed camera under the platform captured the image of each footprint and transmitted the recorded data to the gait analysis software (CatWalk XT, version 10.6; Noldus). Max intensity is the maximum intensity of a paw at maximum contact to the glass platform, ranging from 0 to 255. Single stance is the duration of ground contact by a single paw. Stride length is the distance between successive placements of the same paw.
AAV vectors
The therapeutic AAV vector construct contains codon-optimized human NGLY1 cDNA driven by the CMV/CB promoter. This cassette was packaged into a single-stranded AAV9 and AAV.PHPeB vectors. AAV vectors were produced using standard triple-transfection method and purified by CsCl or iodixanol ultracentrifugation. rAAV titers were determined by droplet digital PCR (for rAAV genome) and gel electrophoresis followed by silver staining (for rAAV capsid).
Quantification of vector genome copies and cDNA by droplet digital PCR
DNA and RNA were extracted from mouse tissues using the AllPrep DNA/RNA Mini Kit (QIAGEN, 80204). RNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, 43-688-13). The endogenous mouse Ngly1 cDNA was quantified in a multiplexed reaction using TaqMan reagents targeting mouse Ngly1 exons 10–11 (Thermo Fisher Scientific, assay ID: Mm01319643_m1) and Gusb (Thermo Fisher Scientific, assay ID: Mm01197698_m1). The vector genome copy number was determined in a multiplexed reaction using TaqMan reagents targeting opt-hNGLY1 (Thermo Fisher Scientific, assay ID: AP329CT) and Tfrc (Thermo Fisher Scientific, 4458367). The opt-hNGLY1 cDNA was quantified in a multiplexed reaction using TaqMan reagents targeting opt-hNGLY1 (Thermo Fisher Scientific, assay ID: AP329CT) and Gusb (Thermo Fisher Scientific, assay ID: Mm01197698_m1).
Quantitation of GNA
GNA in sera and tissues was quantified by Oakland Analytics as previously described (24, 43). Briefly, samples were homogenized in water by bead milling and mixed with 4× volumes of ice-cold internal standard solution (9:1 acetonitrile/water containing 0.05% formic acid and 50 ng/mL GNA-d3). The mixture was centrifuged at 6,100g for 30 minutes, and an aliquot of each supernatant was transferred to an autosampler plate and sealed with a cap-mat. The sample was separated by high-pressure liquid chromatography (Shimadzu VP Series 10 System) and subsequently analyzed using tandem mass spectrometry (Applied Biosystems/MDS SCIEX API 4000).
Statistics
Data were presented as mean ± SD. Comparisons between 2 groups were analyzed by t test (2 sided). Any comparison among multiple groups was analyzed by 1-way or 2-way ANOVA followed by pairwise comparison, corrected for multiple comparisons. All statistical tests were performed with GraphPad Prism 10.
Study approval
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Chan Medical School.
Data availability
Data are available in the Supporting Data Values XLS file.
-
Author contributions
AD, GG, and DW designed the study. AD, KY, XZ, CZ, JL, LR, and NL conducted experiments. KY and NY provided the Ngly1fl/fl iCre mouse line. AD, GG, NY, and DW analyzed the data. AD wrote the original draft. AD, GG, and DW reviewed and edited the manuscript.
-
Acknowledgments
We sincerely thank Tadashi Suzuki (RIKEN) for providing the mice harboring the Ngly1fl allele. We thank the Mouse Behavioral Core and Bone Core of University of Massachusetts Chan Medical School for helping with mouse phenotype assessment. This work was funded in part by Grace Science Foundation. The Wang Lab is supported by a grant from the National Institutes of Health (NIH) (P01HL158506). The Gao Lab is supported by grants from the NIH (R01NS076991-01, P01AI100263-01, P01HL131471-02, 35 R01AI121135, UG3HL147367-01, R01HL097088, and U19AI149646-01) and Cystic Fibrosis Foundation. Some illustrations were created with BioRender.com.
Address correspondence to: Dan Wang or Guangping Gao, Horae Gene Therapy Center, University of Massachusetts Chan Medical School, Worcester, MA 01605, USA. Phone: 774.455.4574; Email: Dan.Wang@umassmed.edu (DW). Phone: 508.856.3563; Email: Guangping.Gao@umassmed.edu (GG).
-
Footnotes
Conflict of interest: GG is a scientific cofounder of Voyager Therapeutics, Adrenas Therapeutics, and Aspa Therapeutics and holds equity in the companies.
Copyright: © 2024, Du et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2024;9(19):e183189.https://doi.org/10.1172/jci.insight.183189.
-
References
- Need AC, et al. Clinical application of exome sequencing in undiagnosed genetic conditions. J Med Genet. 2012;49(6):353–361.
- Caglayan AO, et al. NGLY1 mutation causes neuromotor impairment, intellectual disability, and neuropathy. Eur J Med Genet. 2015;58(1):39–43.
- Heeley J, Shinawi M. Multi-systemic involvement in NGLY1-related disorder caused by two novel mutations. Am J Med Genet A. 2015;167A(4):816–820.
- Lam C, et al. Prospective phenotyping of NGLY1-CDDG, the first congenital disorder of deglycosylation. Genet Med. 2017;19(2):160–168.
- Ge H, et al. Two novel compound heterozygous mutations in NGLY1as a cause of congenital disorder of deglycosylation: a case presentation. BMC Med Genet. 2020;21(1):135.
- Lipinski P, et al. NGLY1 deficiency: novel patient, review of the literature and diagnostic algorithm. JIMD Rep. 2020;51(1):82–88.
- Kariminejad A, et al. NGLY1 deficiency: novel variants and literature review. Eur J Med Genet. 2021;64(3):104146.
- Suzuki T, Yoshida Y. Ever-expanding NGLY1 biology. J Biochem. 2022;171(2):141–143.
- Tong S, et al. NGLY1 deficiency: a prospective natural history study. Hum Mol Genet. 2023;32(18):2787–2796.
- Lam C, et al. NGLY1-related congenital disorder of deglycosylation. In: Adam MP, et al., eds. GeneReviews. Seattle; 1993.
- Enns GM, et al. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. Genet Med. 2014;16(10):751–758.
- Lipari Pinto P, et al. NGLY1 deficiency-A rare congenital disorder of deglycosylation. JIMD Rep. 2020;53(1):2–9.
- Panneman DM, et al. Variants in NGLY1 lead to intellectual disability, myoclonus epilepsy, sensorimotor axonal polyneuropathy and mitochondrial dysfunction. Clin Genet. 2020;97(4):556–566.
- Suzuki T, et al. Ngly1, a mouse gene encoding a deglycosylating enzyme implicated in proteasomal degradation: expression, genomic organization, and chromosomal mapping. Biochem Biophys Res Commun. 2003;304(2):326–332.
- Suzuki T. The cytoplasmic peptide:N-glycanase (Ngly1)-basic science encounters a human genetic disorder. J Biochem. 2015;157(1):23–34.
- Pandey A, Jafar-Nejad H. Tracing the NGLY1 footprints: insights from Drosophila. J Biochem. 2022;171(2):153–160.
- Huang C, et al. Endo-β-N-acetylglucosaminidase forms N-GlcNAc protein aggregates during ER-associated degradation in Ngly1-defective cells. Proc Natl Acad Sci U S A. 2015;112(5):1398–1403.
- Suzuki T, et al. The cytoplasmic peptide:N-glycanase (NGLY1) - Structure, expression and cellular functions. Gene. 2016;577(1):1–7.
- Lehrbach NJ. NGLY1: insights from Caenorhabditis elegans. J Biochem. 2022;171(2):145–152.
- Manole A, et al. NGLY1 mutations cause protein aggregation in human neurons. Cell Rep. 2023;42(12):113466.
- Fujihira H, et al. Physiological importance of NGLY1, as revealed by rodent model analyses. J Biochem. 2022;171(2):161–167.
- Haijes HA, et al. Aspartylglycosamine is a biomarker for NGLY1-CDDG, a congenital disorder of deglycosylation. Mol Genet Metab. 2019;127(4):368–372.
- Hirayama H, Suzuki T. Assay for the peptide:N-glycanase/NGLY1 and disease-specific biomarkers for diagnosing NGLY1 deficiency. J Biochem. 2022;171(2):169–176.
- Mueller WF, et al. GlcNAc-Asn is a biomarker for NGLY1 deficiency. J Biochem. 2022;171(2):177–186.
- Maynard JC, et al. Cytosolic N-GlcNAc proteins are formed by the action of endo-β-N-acetylglucosaminidase. Biochem Biophys Res Commun. 2020;530(4):719–724.
- Fujihira H, et al. Lethality of mice bearing a knockout of the Ngly1-gene is partially rescued by the additional deletion of the Engase gene. PLoS Genet. 2017;13(4):e1006696.
- Bi Y, et al. Repurposing of proton pump inhibitors as first identified small molecule inhibitors of endo-β-N-acetylglucosaminidase (ENGase) for the treatment of NGLY1 deficiency, a rare genetic disease. Bioorg Med Chem Lett. 2017;27(13):2962–2966.
- Kario E, et al. N-linked glycosylation does not impair proteasomal degradation but affects class I major histocompatibility complex presentation. J Biol Chem. 2008;283(1):244–254.
- Tomlin FM, et al. Inhibition of NGLY1 inactivates the transcription factor Nrf1 and potentiates proteasome inhibitor cytotoxicity. ACS Cent Sci. 2017;3(11):1143–1155.
- Yang K, et al. N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1. J Exp Med. 2018;215(10):2600–2616.
- Lehrbach NJ, et al. Protein sequence editing of SKN-1A/Nrf1 by Peptide:N-Glycanase controls proteasome gene expression. Cell. 2019;177(3):737–750.
- Owings KG, et al. Transcriptome and functional analysis in a Drosophila model of NGLY1 deficiency provides insight into therapeutic approaches. Hum Mol Genet. 2018;27(6):1055–1066.
- Singh R, et al. Impact of hybrid and complex N-glycans on cell surface targeting of the endogenous chloride cotransporter Slc12a2. Int J Cell Biol. 2015;2015:505294.
- Talsness DM, et al. A Drosophila screen identifies NKCC1 as a modifier of NGLY1 deficiency. Elife. 2020;9:e57831.
- Galeone A, et al. Tissue-specific regulation of BMP signaling by Drosophila N-glycanase 1. Elife. 2017;6:e27612.
- Galeone A, et al. Regulation of BMP4/Dpp retrotranslocation and signaling by deglycosylation. Elife. 2020;9:e55596.
- Kong J, et al. Mitochondrial function requires NGLY1. Mitochondrion. 2018;38:6–16.
- Han SY, et al. A conserved role for AMP-activated protein kinase in NGLY1 deficiency. PLoS Genet. 2020;16(12):e1009258.
- Tambe MA, et al. N-Glycanase 1 transcriptionally regulates aquaporins independent of its enzymatic activity. Cell Rep. 2019;29(13):4620–4631.
- Dabaj I, et al. NGLY1 deficiency: a rare newly described condition with a typical presentation. Life (Basel). 2021;11(3):187.
- Asahina M, et al. Ngly1 -/- rats develop neurodegenerative phenotypes and pathological abnormalities in their peripheral and central nervous systems. Hum Mol Genet. 2020;29(10):1635–1647.
- Asahina M, et al. Reversibility of motor dysfunction in the rat model of NGLY1 deficiency. Mol Brain. 2021;14(1):91.
- Zhu L, et al. AAV9-NGLY1 gene replacement therapy improves phenotypic and biomarker endpoints in a rat model of NGLY1 deficiency. Mol Ther Methods Clin Dev. 2022;27:259–271.
- Hudry E, Vandenberghe LH. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron. 2019;101(5):839–862.
- Deverman BE, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34(2):204–209.
- Chan KY, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20(8):1172–1179.
- Nonnenmacher M, et al. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol Ther Methods Clin Dev. 2021;20:366–378.
- Stanton AC, et al. Systemic administration of novel engineered AAV capsids facilitates enhanced transgene expression in the macaque CNS. Med. 2023;4(1):31–50.
- Chuapoco MR, et al. Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain. Nat Nanotechnol. 2023;18(10):1241–1251.
- Dyno Therapeutics. Dyno Therapeutics Launches the Dyno bCap 1 Capsid Product, a Breakthrough CNS Gene Delivery Vector Created with Generative Artificial Intelligence. https://www.dynotx.com/bcap1-breakthrough/ Updated May 19, 2023. Accessed August 29, 2024.
- Moyer TC, et al. Highly conserved brain vascular receptor ALPL mediates transport of engineered viral vectors across the blood-brain barrier [preprint]. https://doi.org/101101/20240312584703 Posted on bioRxiv March 14, 2024.
- Jackson KL, et al. AAV9 supports wide-scale transduction of the CNS and TDP-43 disease modeling in adult rats. Mol Ther Methods Clin Dev. 2015;2:15036.
- Jackson KL, et al. Better targeting, better efficiency for wide-scale neuronal transduction with the synapsin promoter and AAV-PHP.B. Front Mol Neurosci. 2016;9:116.
- Dayton RD, et al. More expansive gene transfer to the rat CNS: AAV PHP.EB vector dose-response and comparison to AAV PHP.B. Gene Ther. 2018;25(5):392–400.
- Fujihira H, et al. Liver-specific deletion of Ngly1 causes abnormal nuclear morphology and lipid metabolism under food stress. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3):165588.
- Foust KD, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65.
- Duque S, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17(7):1187–1196.
- Hoy SM. Onasemnogene abeparvovec: first global approval. Drugs. 2019;79(11):1255–1262.
- Thomsen G, et al. Biodistribution of onasemnogene abeparvovec DNA, mRNA and SMN protein in human tissue. Nat Med. 2021;27(10):1701–1711.
- Ling Q, et al. AAV-based in vivo gene therapy for neurological disorders. Nat Rev Drug Discov. 2023;22(10):789–806.
- Mathiesen SN, et al. CNS transduction benefits of AAV-PHP.eB over AAV9 are dependent on administration route and mouse strain. Mol Ther Methods Clin Dev. 2020;19:447–458.
- Goertsen D, et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat Neurosci. 2022;25(1):106–115.
- Matsuzaki Y, et al. Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci Lett. 2018;665:182–188.
- Luoni M, et al. Whole brain delivery of an instability-prone Mecp2 transgene improves behavioral and molecular pathological defects in mouse models of Rett syndrome. Elife. 2020;9:e52629.
- Chadman KK, et al. Efficient selivery of FMR1 across the blood brain barrier using AAVphp construct in adult FMR1 KO mice suggests the feasibility of gene therapy for fragile X syndrome. Genes (Basel). 2023;14(2):505.
- Valassina N, et al. Scn1a gene reactivation after symptom onset rescues pathological phenotypes in a mouse model of Dravet syndrome. Nat Commun. 2022;13(1):161.
- Davidson CD, et al. Improved systemic AAV gene therapy with a neurotrophic capsid in Niemann-Pick disease type C1 mice. Life Sci Alliance. 2021;4(10):e202101040.
- Lim JA, et al. Intravenous injection of an AAV-PHP.B vector encoding human acid βα-glucosidase rescues both muscle and CNS defects in murine Pompe Disease. Mol Ther Methods Clin Dev. 2019;12:233–245.
- Morabito G, et al. AAV-PHP.B-mediated global-scale expression in the mouse nervous system enables GBA1 gene therapy for wide protection from synucleinopathy. Mol Ther. 2017;25(12):2727–2742.
- Leborgne C, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med. 2020;26(7):1096–1101.
- Elmore ZC, et al. Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight. 2020;5(19):e139881.
- Duan D. Lethal immunotoxicity in high-dose systemic AAV therapy. Mol Ther. 2023;31(11):3123–3126.
- Huang Q, et al. An AAV capsid reprogrammed to bind human transferrin receptor mediates brain-wide gene delivery. Science. 2024;384(6701):1220–1227.
- Hughes LA, et al. Copy number variation in tRNA isodecoder genes impairs mammalian development and balanced translation. Nat Commun. 2023;14(1):2210.
- de Boer J, et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296(5571):1276–1279.
- Laws N, Hoey A. Progression of kyphosis in mdx mice. J Appl Physiol (1985). 2004;97(5):1970–1977.
- Guyenet SJ, et al. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J Vis Exp. 2010;39:1787.
- Walter J, et al. The CatWalk XT is a valid tool for objective assessment of motor function in the acute phase after controlled cortical impact in mice. Behav Brain Res. 2020;392:112680.
-
Version history
- Version 1 (August 13, 2024): In-Press Preview
- Version 2 (October 8, 2024): Electronic publication











