Research LetterGastroenterologyImmunologyInflammation Free access | 10.1172/jci.insight.192629
A vaccination strategy to prevent coxsackie virus B3–induced development of pancreatic cancer
Veethika Pandey, Heike R. Döppler, Ligia I. Bastea, Alicia K. Fleming Martinez, Barath Shreeder, Brandy H. Edenfield, Keith L. Knutson, DeLisa Fairweather, and Peter Storz
1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by Pandey, V. in: PubMed | Google Scholar
1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by Döppler, H. in: PubMed | Google Scholar
1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by Bastea, L. in: PubMed | Google Scholar
1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by Fleming Martinez, A. in: PubMed | Google Scholar
1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by Shreeder, B. in: PubMed | Google Scholar
1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by Edenfield, B. in: PubMed | Google Scholar
1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by Knutson, K. in: PubMed | Google Scholar
1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by
Fairweather, D.
in:
PubMed
|
Google Scholar
|

1Department of Cancer Biology,
2Immunology Department, and
3Department of Cardiovascular Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Address correspondence to: Peter Storz, Mayo Clinic, 4500 San Pablo Road, Jacksonville, Florida 32224, USA. Phone: 904.953.6909; Email: storz.peter@mayo.edu.
Find articles by Storz, P. in: PubMed | Google Scholar
Published July 31, 2025 - More info
JCI Insight. 2025;10(18):e192629. https://doi.org/10.1172/jci.insight.192629.
© 2025 The American Society for Clinical Investigation
-
To the editor:
Coxsackie virus B (CVB) infections are a common occurrence in human pancreatic ductal adenocarcinoma (PDAC). Of the 6 CVB serotypes (CVB1–6), infection with CVB3 shows an incidence of approximately 50% (1). Preclinical data with the precancerous p48Cre LSL-KrasG12D (KC) animal model indicated that a one-time infection with CVB3 accelerates pancreatic cancer development by generating a prolonged desmoplastic reaction (1). This suggests that preventing CVB infection could be an efficient strategy to block PDAC from occurring. This was tested here by vaccination with two CVB3 capsid peptides, which blocked CVB3 effects and kept pancreatic lesions at a precancerous stage.
We s.c. inoculated a mixture of 2 linear peptides (VP1-1, GPVEDAITAAIGRVA; VP1-24, IKSTIRIYFKPKHVK), both part of the VP1 subunit of the viral coat protein (capsid) and immunogenic (2) into (4-week-old) KC mice over a period of 4 weeks (Figure 1A). Submandibular blood samples were collected at days 14 and 28 and were tested for serum antibodies directed against CVB3 capsid using ELISA. We detected a strong immune response to the peptide mix during the vaccination period (Figure 1B). Therefore, a 6-time s.c. inoculation of these peptides in mice over 4 weeks was concluded to be an efficient way to vaccinate against CVB3.
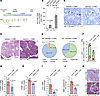 Figure 1
Figure 1A preclinical proof-of-principle experiment demonstrating that vaccination prevents CVB3-induced development of pancreatic cancer. (A) Timeline. (B) CVB3 capsid antibodies in blood samples at days 14 and 28 in the 4-week vaccination period via ELISA. (C) Representative pictures of IHC staining for CVB3 at endpoint. Arrows indicate CVB3+ cells. Scale bar: 100 μm. (D) H&E staining of a representative area of the pancreas. Scale bar: 1 mm. (E) Quantification of ADM, PanIN1A/B, PanIN2, and PanIN3 in CVB3 infected KC mice either vaccinated (n = 7) or vehicle treated (n = 5). P values for vehicle versus vaccinated CVB3-treated KC mice: ADM, P < 0.0001; PanIN1A/B, P < 0.0001; PanIN2, P < 0.0001. (F) Bar graph indicating the percentage of CD3+CD4+ and CD3+CD8+ T cells. (G–J) Bar graphs indicating relative change of Treg (FoxP3+ T cells), total macrophages (F4/80+ cells), Ym1+ macrophages, or SMA+ stellate cells between conditions. (K) Representative pictures of trichrome staining for n = 5 for both conditions. Scale bar: 100 μm. In B and E–J, the statistical significance between groups was determined using a 2-tailed t test. In B and F–J, data are shown as mean ± SD, and individual dots represent samples from individual mice.
To test if vaccination produces neutralizing serum antibodies that protect from CVB3 infection, we performed a 1-time infection with CVB3 after the end of the vaccination period. Six weeks after infection, mice were euthanized and pancreatic tissue was analyzed (Figure 1A). We observed substantial decrease in CVB3+ cells in vaccinated KC mice, when compared with vehicle-treated mice (Figure 1C and Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.192629DS1), indicating protection of pancreatic tissue against viral infection. H&E staining revealed a decrease in pancreatic abnormal areas (lesions and microenvironment) in vaccinated KC mice (Figure 1D and Supplemental Figure 1B). Pancreatic lesions (Figure 1E) in vaccinated CVB3-infected KC mice consisted mainly of acinar-to-ductal metaplasia (ADM; 64% ± 7.4%) and low-grade PanIN1 (35% ± 5.5%). This is comparable with our published data on lesions in untreated KC mice of the same age (ADM, 67% ± 4.9%; PanIN1, 32.3% ± 4.6%; ref. 1) and indicates that CVB3 infection was successfully blocked. Pancreata of vehicle-injected, CVB3-infected KC mice showed enhanced progression as indicated by a decrease in ADM (21% ± 8.2%) correlating with an increase in PanIN1 (72% ± 6.4%) and PanIN2 (6% ± 1.1%), and even sporadic (1% ± 0.5%) presence of carcinoma in situ (PanIN3).
We observed an altered immune environment after vaccination. While cytotoxic (CD3+CD8+) T cells are found in low numbers in control or CVB3-infected pancreata, regulatory (CD3+CD4+) T cells, which have been linked to PDAC development, dramatically increase in numbers after CVB3 infection (1, 3). We here show that our vaccination strategy decreases the highly-abundant CD4+ T cell populations (Figure qF and Supplemental Figure 2A), from 79% ± 8.4% of the total T cell population to 43% ± 15.8%. These include immunosuppressive (FoxP3+) Tregs (Figure 1G and Supplemental Figure 2B).
CVB3 infection in pancreata of KC mice leads to an increase in neutrophils and macrophages in the lesion microenvironment (1). After vaccination, Ly6B.2+ neutrophils decreased by an average of 60.5% (Supplemental Figure 1C). Total macrophages decreased about 25% (Figure 1H and Supplemental Figure 2C). Of these, Ym1+ alternatively activated macrophages (AAM), which have been shown to activate stellate cells and contribute to fibrosis (4, 5), were decreased to 20.3% after vaccination (Figure 1I and Supplemental Figure 2D). Vaccinated CVB3-infected mice showed less-activated stellate cells (SMA+)(Figure 1J and Supplemental Figure 2E), and trichrome staining confirmed a decrease in the fibrotic response around lesions (Figure 1K).
In conclusion, our data provide a preclinical proof-of-principle experiment demonstrating that vaccination against CVB viral infections could be a valuable strategy to prevent pancreatic cancer. However, since all 6 CVB serotypes have been detected in human pancreatic cancer (1), a successful vaccination strategy for patients will need to prevent infections with all serotypes. Such vaccinations would be of benefit for individuals at age 70 or higher as a high-risk group since PanIN and other ductal lesions are found inj approximately 80% of this population, and approximately 95% of these lesions have oncogenic mutations in KRAS.
Our vaccination approach generates neutralizing antibodies and, therefore, should prevent disease. Approximately 60% of healthy donors have neutralizing serum antibodies for CVB3 due to previous exposure. However, it is unclear for how long neutralizing antibodies are present after infection. This information is needed to determine if a 1-time vaccination is efficient or if more frequent vaccinations will be needed. This will be rigorously addressed with future animal studies and analyses of epidemiology data. Future studies also will aim to identify potential additional immunomodulatory effects induced by the adjuvant, and they need to address the long-term effects on survival of CVB3-infected KC mice following vaccination.
For detailed methods, information regarding sex as a biological variable, statistics, study approval, data availability, author contributions, and acknowledgments, see the Supplemental Methods.
-
Footnotes
Conflict of interest: The authors have declared that no conflict interest exists.
Copyright: © 2025, Pandey et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Submitted: February 19, 2025
Reference information: JCI Insight. 2025;10(18):e192629. https://doi.org/10.1172/jci.insight.192629.
-
References
- Bastea LI, et al. Coxsackievirus and adenovirus receptor expression facilitates enteroviral infections to drive the development of pancreatic cancer. Nat Commun. 2024;15(1):10547.
- Haarmann CM, et al. Identification of serotype-specific and nonserotype-specific B-cell epitopes of coxsackie B virus using synthetic peptides. Virology. 1994;200(2):381–389.
- Kemball CC, et al. Enumeration and functional evaluation of virus-specific CD4+ and CD8+ T cells in lymphoid and peripheral sites of coxsackievirus B3 infection. J Virol. 2008;82(9):4331–4342.
- Fleming Martinez AK, et al. Ym1+ macrophages orchestrate fibrosis, lesion growth, and progression during development of murine pancreatic cancer. iScience. 2022;25(5):104327.
- Liou GY, et al. The presence of interleukin-13 at pancreatic ADM/PanIN lesions alters macrophage populations and mediates pancreatic tumorigenesis. Cell Rep. 2017;19(7):1322–1333.
-
Version history
- Version 1 (July 31, 2025): In-Press Preview
- Version 2 (September 23, 2025): Electronic publication




