Research ArticleMetabolismNephrology
Open Access |  10.1172/jci.insight.190992
10.1172/jci.insight.190992
Distinct cell types along thick ascending limb express pathways for monovalent and divalent cation transport
Hasan Demirci,1,2,3 Jessica P. Bahena-Lopez,4 Alina Smorodchenko,5 Xiao-Tong Su,4 Jonathan W. Nelson,6 Chao-Ling Yang,4 Joshua N. Curry,4 Xin-Peng Duan,7,8 Wen-Hui Wang,8 Yuliya Sharkovska,9 Ruisheng Liu,10 Duygu Elif Yilmaz,1,11 Catarina Quintanova,12 Katie Emberley,13 Ben Emery,13 Nina Himmerkus,12 Markus Bleich,12 David H. Ellison,4 and Sebastian Bachmann1,2
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by
Demirci, H.
in:
PubMed
|
Google Scholar
|

1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Bahena-Lopez, J. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Smorodchenko, A. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by
Su, X.
in:
PubMed
|
Google Scholar
|

1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Nelson, J. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Yang, C. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by
Curry, J.
in:
PubMed
|
Google Scholar
|

1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by
Duan, X.
in:
PubMed
|
Google Scholar
|

1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Wang, W. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Sharkovska, Y. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by
Liu, R.
in:
PubMed
|
Google Scholar
|

1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by
Yilmaz, D.
in:
PubMed
|
Google Scholar
|

1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Quintanova, C. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Emberley, K. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by
Emery, B.
in:
PubMed
|
Google Scholar
|

1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Himmerkus, N. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by
Bleich, M.
in:
PubMed
|
Google Scholar
|

1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Ellison, D. in: PubMed | Google Scholar
1Institute of Functional Anatomy and
2Department of Cell Biology and Neurobiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
3Department of Medical Biology, Faculty of Medicine, Ankara Yildirim Beyazit University, Ankara, Turkey.
4Division of Nephrology and Hypertension, School of Medicine, Oregon Health & Science University, Portland, Oregon, USA.
5Department of Human Medicine, MSB Medical School Berlin, Berlin, Germany.
6Division of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, USA.
7Department of Physiology, Xuzhou Medical University, Xuzhou, China.
8Department of Pharmacology, New York Medical College, Valhalla, New York, New York, USA.
9Department of Pediatric Respiratory Medicine and Department for Pediatric Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
10Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida, USA.
11Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
12Institute of Physiology, Christian-Albrechts-University, Kiel, Germany.
13Department of Neurology, Jungers Center for Neurosciences Research, Oregon Health & Science University, Portland, Oregon, USA.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Find articles by Bachmann, S. in: PubMed | Google Scholar
Authorship note: HD and JPBL contributed equally to this work. DHE and SB are equal corresponding authors.
Published June 5, 2025 - More info
JCI Insight. 2025;10(13):e190992. https://doi.org/10.1172/jci.insight.190992.
© 2025 Demirci et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Received: January 15, 2025; Accepted: May 28, 2025
Related video:
Pathways for monovalent and divalent cation transport and distinct cell types
In this episode, David Ellison, Sebastian Bachman, and Jessica Bahena-Lopez describe how their results show that there are three cell types along the kidney thick ascending limb, enabling it to adjust solute absorption selectively to maintain homeostasis.
-
Abstract
Kidney thick ascending limb (TAL) cells reabsorb sodium, potassium, calcium, and magnesium and contribute to urinary concentration. These cells are typically viewed as a single type that recycles potassium across the apical membrane and generates a lumen-positive transepithelial voltage driving calcium and magnesium reabsorption, though variability in potassium channel expression has been reported. Additionally, recent transcriptomic analyses suggest that different cell types exist along this segment, but classifications have varied and have not led to a new consensus model. We used immunolocalization, electrophysiology, and enriched single-nucleus RNA-Seq to identify TAL cell types in rats, mice, and humans. We identified 3 major TAL cell types defined by expression of potassium channels and claudins. One has apical potassium channels, has low basolateral potassium conductance, and is bordered by a monovalent cation-permeable claudin. A second lacks apical potassium channels, has high basolateral potassium conductance, and is bordered by calcium- and magnesium-permeable claudins. A third type also lacks apical potassium channels and has high basolateral potassium conductance, but these cells are ringed by monovalent cation-permeable claudins. The recognition of diverse cell types may resolve longstanding questions about how solute transport can be modulated selectively and how disruption of these cells leads to human disease.
-
Introduction
The kidney thick ascending limb (TAL) reabsorbs several filtered solutes and contributes importantly to urinary concentration and dilution. As physiological challenges to extracellular fluid volume, divalent ion metabolism, or water balance may occur independently, the TAL must be able to adjust solute transport selectively in response to physiological need. Yet, TAL cells are generally viewed as expressing a common set of key transport proteins. These include the sodium, potassium, chloride transporter (NKCC2) and a K+ channel (ROMK) at the apical membrane and a chloride channel at the basolateral membrane. The combination of K+ recycling across the apical membrane and Cl– exit across the basolateral membrane generates a transepithelial voltage oriented with the lumen positive relative to interstitium (1–3).
Ca2+ and Mg2+ reabsorption along the TAL take place via a paracellular pathway comprising claudins 16 and 19 (4), driven by the transepithelial voltage. Transport of these ions can be modulated separately from that of Na+. High Ca2+ intake, for example, decreases paracellular Ca2+ transport along the cortical TAL (cTAL) without altering transcellular NaCl transport (5), an effect that is likely mediated in part by the calcium-sensing receptor (CaSR) (6). While activating mutations of the CaSR in humans sometimes lead to mild salt wasting (7), the bulk of evidence indicates that CaSR activation along the TAL primarily affects Ca2+ transport with minimal effects on transepithelial voltage or NaCl excretion (6, 8). Along the same lines, parathyroid hormone (PTH) appears capable of stimulating Ca2+ reabsorption with little or no effect on transepithelial voltage or NKCC2 (9).
One way that differential regulation may occur is because the TAL comprises different cell types. Early studies in amphiuma and hamster posited the existence of 2 cell types, one with high basolateral K+ conductance (HBC) and another with low basolateral conductance (LBC) (10, 11). It was later shown that some TAL cells do not express ROMK at their apical membrane (12, 13), a finding incorporated by Dimke and Schnermann in a TAL model with 2 cell types based on K+ conductance (14). Yet, their model did not link K+-conductive cell types to cells that transport Ca2+ and Mg2+.
More recently, Milatz and colleagues (15) reported that different claudins, tight junction proteins that act as paracellular solute “gates,” are expressed in a mutually exclusive mosaic pattern along the TAL. As these claudins are selectively permeable for divalent (claudins 16 and 19, CLDN16 and CLDN19) and monovalent cations (claudin 10, CLDN10), this suggested that there are discrete paracellular pathways for the different cations (4). Recently, transcriptomic analyses have also detected clusters of TAL cells, one predominantly expressing CLDN16 and the other predominantly expressing CLDN10 (16–18), seemingly aligning with the immunolocalization results. Yet the terms used to denote cells and the gene signatures involved (17–20) have not been integrated with important, but often neglected, earlier results concerning sodium and potassium transport pathways (10–13). Here, by combining immunocytochemical, transcriptomic, and electrophysiological approaches across multiple species, we show the existence of 2 primary TAL cell types in the cortex and outer stripe of the outer medulla (OSOM), one that should transport Na+ efficiently and generate the transepithelial voltage and the other that should be electroneutral, transport Ca2+ and Mg2+, and respond to regulators of Ca2+ metabolism. A third cell type, present only in the inner stripe of the outer medulla (ISOM), combines key features of the other two and likely participates in K+ reabsorption.
-
Results
Immunohistochemistry reveals mosaicism of ion channel expression. We first verified that, in contrast with uniform expression of NKCC2 along the entire TAL, apical immunoreactive ROMK signal shows mosaic expression along both cTAL and medullary TAL (mTAL) (Figure 1A and Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.190992DS1). ROMK signal was concentrated in the apical aspect of cells categorized as positive with primary antibody concentration adjusted to optimal signal-to-noise ratio. In this standard condition, cells categorized as negative were lacking detectable luminal ROMK signal and were distributed singly or in patches of 2 to 4 cells. At electron microscopic resolution, ROMK immunoreactive signal was strictly located at the apical membrane of ROMK-positive TAL cells by immunoperoxidase staining; the signal ended abruptly at the tight junctions coupling ROMK-positive to adjacent ROMK-negative cells (Figure 1, B and C). cTAL segments contained 28% ± 2% ROMK-negative cells, mTAL of OSOM 37% ± 1%, and mTAL of ISOM 46% ± 7%, respectively (Figure 1D). Mosaic ROMK signal was comparable across species with mouse and rat kidney samples displaying similar numerical outcome; no significant sex differences were encountered. Concomitant NKCC2 apical signal was uniform, lacking mosaicism throughout (Supplemental Figure 1, B–D). In contrast with the mosaicism of ROMK protein signal, Kcnj1 mRNA, encoding ROMK, was present in all TAL cells as detected by in situ hybridization (Supplemental Figure 2).
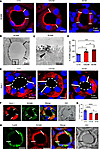 Figure 1
Figure 1Immunostaining for ROMK, Kir4.1, and CaSR distribution in rat kidney TAL. (A) ROMK immunostaining is identified using an antibody that recognizes all 3 ROMK isoforms (see also Supplemental Table 1) by luminal signal in a subset of cells lining TAL profiles across renal parenchymal zones; DAPI blue nuclear staining. White bars in epithelia identify cell borders between ROMK-positive and ROMK-negative cells. (B and C) Transmission electron microscopy showing overview (B) and detail (C) of ROMK-positive and ROMK-negative adjacent cells; arrowhead marks junctional area between positive and negative cells (C); immunoperoxidase staining. Scale bars indicated. (D) Numerical evaluation of ROMK-negative cells across the zones; values are means ± SD from n = 5 rats; *P < 0.05; ***P < 0.001, by 1-way ANOVA and post hoc comparisons. (E) Kir4.1 immunostaining is identified as basolateral signal in a subset of medullary TAL (mTAL) epithelial cells. (F) Double immunostaining for Kir4.1 and ROMK shows mutually exclusive signals in mTAL. (G) Numerical evaluation of Kir4.1-negative cells across zones; values are means ± SD from n = 4 rats; ****P < 0.0001, by 1-way ANOVA and post hoc comparisons. (H) Double immunostaining for CaSR and ROMK show mutually exclusive signals in mTAL. White bars mark the borders between ROMK-positive and -negative cells. To reflect underlying structure, a differential interference contrast (DIC) filter was used. DAPI blue nuclear staining: scale bars indicated.
The Kir4.1 inwardly rectifying K+ channel, known to be present along the basolateral membrane of TAL cells (21), revealed mosaicism as well that was mutually exclusive to ROMK expression in TAL across zones. Commonly, ROMK-negative TAL cells thus expressed significant Kir4.1 signal, whereas ROMK-positive cells were devoid of Kir4.1 signal (Figure 1, E and F). Kir4.1-negative cells amounted to 48% ± 8% in cTAL, 36% ± 6% in mTAL of OSOM, and 33% ± 7% in mTAL of ISOM (Figure 1G). Further mosaicism was detected for the CaSR with strong signal in cTAL and gradually decreasing strength in mTAL. Cells expressing either CaSR or ROMK were typically found in mTAL, whereas in cTAL, ROMK-positive cells commonly also expressed CaSR (Figure 1H).
Potassium channels in macula densa. In macula densa, ROMK signal was evenly or irregularly distributed (Supplemental Figure 3). ROMK-negative cells were mostly solitary, but 2 or 3 adjacent negative cells were also found (Supplemental Figure 3). In the postmacula segment, ROMK distribution did not differ from that in upstream cTAL.
ROMK is expressed in cells with lower total potassium conductance. The immunolocalization studies suggest differential K+-conductive pathways among TAL cells, and as 2 patterns of TAL cell conductances were reported in hamster (11), we performed whole-cell voltage-clamp experiments in isolated and split-open mouse cTAL. Figure 2A shows examples of patch-clamp recordings from tertiapin-Q–sensitive (TPNQ, 400 nM) K+ currents indicating ROMK at –40 mV (22). The residual Ba2+-sensitive K+ currents indicate K+ channels other than ROMK. Figure 2B summarizes the results, showing that there are 2 types of cTAL cells, one displaying TPNQ-sensitive ROMK channel activity (390 ± 42 pA; 6 of 14 cells) and another with no ROMK channel activity (8 of 14 cells); the latter display higher Ba2+-sensitive K+ currents compared with the former. As whole-cell electrophysiology does not permit luminal and basolateral membranes to be perturbed individually, we perfused dissected cTAL segments. Membrane voltage changes were measured by confocal microscopy using the membrane voltage dye Di-8-ANEPPS (Figure 2C). At constant luminal K+ concentration, the basolateral K+ concentration was increased to depolarize cells that have a high fractional K+ conductance at the basolateral membrane. During this maneuver, some TAL cells depolarized, whereas others did not. Relative fluorescence intensities before and after exposure to basolateral 30 mmol/L K+ are shown in Figure 2D from 11 individual cells of 4 TAL tubules. Fluorescence increase indicates depolarization in the presence of 30 mmol/L K+ at the basolateral side, which is indicative of a basolateral K+ conductance. No response indicates that the membrane voltage completely depends on luminal K+ channels as the luminal K+ concentration remained constant throughout the experiment; distinct cell types could thus be identified (Figure 2E).
![Functional evidence for distinct cell types with or without ROMK activity that respond to basolateral [K+]. Functional evidence for distinct cell types with or without ROMK activity t](//df6sxcketz7bb.cloudfront.net/manuscripts/190000/190992/small/jci.insight.190992.f2.gif) Figure 2
Figure 2Functional evidence for distinct cell types with or without ROMK activity that respond to basolateral [K+]. (A) Whole-cell recordings show tertiapin-Q–sensitive (TPNQ-sensitive) K+ currents at –40 mV and barium-sensitive (Ba2+-sensitive) K+ currents at the same voltage in 2 representative TAL cells. Red arrows indicate addition of 400 nM TPNQ to the bath; blue arrows indicate addition of 1 mM Ba2+ to the bath. (B) Dot plot summary of experiments of TPNQ-sensitive and Ba2+-sensitive K+ currents at –40 mV with whole-cell recording. There are 2 types of cTAL cells, one (6 out of 14 cells) with TPNQ-sensitive ROMK channel activity (390 ± 42 pA) and the other (8 out of 14 cells) with no ROMK channel activity. Note that ROMK-negative cells have greater Ba2+-sensitive K+ currents than ROMK-positive cells. Results were obtained from 14 experiments (tubules). (C) Confocal image of an isolated perfused TAL after Di-8-ANEPPS loading. Fluorescence intensity corresponds to membrane voltage and dye concentration. (D) Relative intensity of 11 individual cells from 4 TAL tubules under control (3.6 mmol/L) or high basolateral K+ concentration (30 mmol/L). Note that several lines showing tubules that did not respond to [K+] are superimposed. (E) Relative intensities in the presence of high basolateral K+ concentration suggesting distinct cell types.
Immunohistochemistry links claudin mosaicism with ROMK. Mosaicism was further verified in the expression of the 2 key tight junctional proteins, CLDN10 and CLDN16, along TAL as previously reported (15, 23). Accordingly, CLDN10-positive cells were encountered along the entire cTAL and mTAL, whereas CLDN16-positive cells were restricted to TAL within cortex, OSOM, and outermost ISOM (Figure 3). At the cellular level, CLDN10 immunoreactivity decorated the basolateral membrane and tight junction, as reported (24), whereas CLDN16 was strictly confined to the junctional location. In cortex and OSOM, ROMK-negative cells were entirely surrounded by a CLDN16-positive junctional belt, whereas ROMK-positive cells revealed 2 types of junctional belt composition. In one, ROMK-positive cells surrounded by other ROMK-positive cells expressed a CLDN10 junctional belt at their entire circumference. In the other, ROMK-positive cells bordering ROMK-negative cells displayed CLDN16 junctional belt where they were contacting each other, so that ROMK-positive cells, but not ROMK-negative/Kir4.1-positive cells, revealed a heterogeneity between CLDN10 and CLDN16 signals along their junctional portions (Figure 3, A and B, diagrammed in Figure 3C).
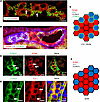 Figure 3
Figure 3Immunostaining for claudin 10, claudin 16, ROMK, and Kir4.1 in rat kidney. Images were taken in subluminal tangential plane of the tubules. (A) Double immunostaining for CLDN16 and ROMK in mTAL of OSOM; note absence of CLDN16 signal between ROMK-positive cells but its presence when lining ROMK-negative cells (arrows). (B) Triple immunostaining for ROMK, Kir4.1, and CLDN16 in cTAL; red and purple signals distinguish ROMK-positive from ROMK-negative/Kir4.1-positive cells, respectively. Note absence of CLDN16 from junctions between ROMK-positive cells but its presence between ROMK-negative/Kir4.1-positive cells. (C) Schematic drawing demonstrates the differences with respect to ROMK-positive, Kir4.1-negative as well as ROMK-negative, Kir4.1-positive cells and their CLDN10- or CLDN16-specific junctional lining in TAL of cortex and OSOM. (D) Double immunostaining for CLDN10 and ROMK in mTAL of ISOM; junctional CLDN10 signals (arrows) between ROMK-positive cells. (E) Double immunostaining for CLDN10 and Kir4.1 in mTAL of ISOM; tight junctions between Kir4.1-positive cells (arrows) express CLDN10. (F) Schematic drawing demonstrates ROMK-positive, Kir4.1-negative as well as ROMK-negative, Kir4.1-positive cells and their CLDN10-specific junctional lining in TAL of ISOM. Scale bars indicated.
These junctional staining patterns were verified in mouse isolated cTAL segments (Supplemental Figure 4), where mixing of CLDN10 and CLDN16 signals in a single tight junction was not observed. Some features in the ISOM were different. As noted above, cells in this region expressed only CLDN10, and this was true for both ROMK-positive and Kir4.1-positive cells (Figure 3, D and E, diagrammed in Figure 3F).
Enriched single-nucleus RNA sequencing reveals distinct TAL cell types. To classify TAL cell types and complement the anatomical and functional studies above, we enriched TAL nuclei for single-nucleus RNA sequencing (snRNA-Seq). Given that TAL cells constitute a small fraction of kidney tissue and exhibit heterogeneity across and within kidney zones, we employed an enrichment approach to enhance resolution. First, we generated NKCC2-INTACT (Isolation of Nuclei TAgged in specific Cell Types) mice by crossing NKCC2-Cre and CAG-Sun1/superfolder GFP (sfGFP) lines, enabling inducible TAL-specific nuclear labeling, as we previously described for distal convoluted tubule (DCT) snRNA-Seq (25). Tamoxifen-induced recombination efficiently targeted TAL cells (Figure 4A). Transcriptomic analysis of enriched NKCC2-INTACT nuclei showed uniform Slc12a1 (NKCC2) expression, validating TAL cell identity (Supplemental Figure 5A). Additionally, kidneys were manually dissected into cortical and medullary regions prior to sequencing, with uniform manifold approximation and projection (UMAP) visualization revealing clear segregation of TAL cells between these regions (Supplemental Figure 5, B and C).
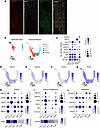 Figure 4
Figure 4TAL cell diversity revealed by enriched snRNA-Seq analysis in the mouse kidney. (A) Immunofluorescence staining of NKCC2-INTACT mouse kidneys shows total NKCC2 (tNKCC2) (red), tNCC (white), and GFP (green). Tamoxifen-induced recombination specifically labels NKCC2+ cells, with GFP-positive nuclei colocalizing exclusively in NKCC2-expressing cells. (B) Uniform manifold approximation and projection (UMAP) of enriched snRNA-Seq data showing TAL cell populations split by kidney region (cortex and medulla). Identified clusters include TAL-α (claudin 10–positive, Kir4.1–negative), TAL-β (claudin 16–positive, Kir4.1–positive), TAL-γ (claudin 10–positive, Kir4.1–positive), macula densa (MD; Nos1–positive), and proliferating (Prolif; Top2a–positive) cells. (C) Dot plot displaying key TAL cell type markers, including Slc12a1, Cldn10, Cldn16, Kcnj10, Kcnj1, Nos1, Top2a. UMAP displaying the expression of Cldn10 (D), Cldn16 (E), Kcnj10 (F), and Kcnj1 (G) genes. Dot plots and heatmaps illustrate gene expression patterns associated with (H) sodium transport (Cldn10, Wnk4, Wnk1, Stk39, Ptger3, Avpr2), which are enriched in TAL-α and TAL-γ clusters; (I) calcium and magnesium transport (Cldn16, Cldn19, Casr, Pth1r, Vdr, Cnnm2, Cldn14), with higher expression in TAL-β; (J) potassium transport (Kcnma1, Kcnj1, Kcnj10, Kcnj16, Kcnt1). Data normalization and scaling: Gene expression data were normalized and z-score–scaled to enable comparison of relative expression levels across clusters. “Avg Exp” (average expression) and “Exp” (expression) represents the z-scored mean gene expression within a cluster, while “Pct Exp” (percentage expressed) denotes the percentage of cells in a cluster with detectable expression of the gene.
Results identified 3 major and 2 minor TAL cell clusters (Figure 4B). The major clusters were classified as TAL-α (claudin 10–positive, Kir4.1-negative), TAL-β (claudin 16–positive, Kir4.1-positive), and TAL-γ (claudin 10–positive, Kir4.1-positive) (Figure 4C and Supplemental Table 1). TAL-α and TAL-β cells were present in both cortical and medullary regions, whereas TAL-γ cells were exclusive to the medulla (Figure 4B). Among the minor clusters, one exhibited nitric oxide synthase 1 (Nos1) expression, indicating that it represents MD cells, while the other expressed DNA topoisomerase II alpha (Top2a), representing a small population of proliferating cells (Figure 4C). These findings align with prior transcriptomic analyses of cortex (17, 18) but provide enhanced resolution, particularly within the medullary regions.
The enriched-TAL dataset revealed molecular heterogeneity, particularly within the medullary TAL. Notably, Kcnj10 (Kir4.1) showed distinct patterns: In the cortex, it was exclusively expressed in claudin 16–positive (TAL-β) cells, while in the medulla, it was expressed in both claudin 16–positive (TAL-β) and claudin 10–positive (TAL-γ) cells (Figure 4, B and D–F, and Supplemental Figure 12). Present immunostaining and previous studies (28) suggested that medullary TAL-β cells reside in the OSOM, whereas TAL-γ cells are likely restricted to the ISOM.
Although Kcnj1 (ROMK) transcripts were detected across all clusters, as noted above (Supplemental Figure 2), differential gene expression analysis revealed significant enrichment in TAL-α (adjusted P = 3.0 × 10–45; Supplemental Data 1), corresponding to apical ROMK expression (Figure 4G). Notably, complete cell type identification requires apical ROMK localization, though transcriptomic data alone can be used to identify TAL-α, TAL-β, and TAL-γ cells (Supplemental Figure 12).
Differentially expressed gene (DEG) analysis further revealed functional distinctions among TAL subtypes. TAL-α and TAL-γ cells (claudin 10–positive) exhibited higher expression of genes associated with sodium transport (Wnk4, Stk39 [SPAK], Avpr2), as well as arachidonic acid signaling (Ptger3), and the organic anion transmembrane transporter Slco3a1 (Oatp3a1), which has been implicated in prostaglandin transport (26) (Figure 4H and Supplemental Figure 6A). In contrast, TAL-β cells (claudin 16–positive) showed enriched expression of genes involved in calcium and magnesium homeostasis, including claudin 19 (Cldn19), claudin 14 (Cldn14), Casr, the PTH receptor (Pth1r), the vitamin D receptor (Vdr), and cyclin and CBS domain divalent metal cation transport mediator 2 (Cnnm2) (Figure 4I). Notably, Wnk1 expression was enriched in TAL-β cells, whereas Wnk4 was more highly expressed in TAL-α cells (Figure 4H).
Other potassium channels also exhibited differential expression. Kcnma1, which encodes the potassium calcium-activated channel subfamily M alpha 1 (BK channel), was predominantly expressed in TAL-α cells, while Kcnj16 (Kir5.1), a channel that forms heterotetramers with Kir4.1, was widely expressed across TAL types but with highest expression in TAL-β cells (Figure 4J). Kcnt1, recently linked to the 70 pS apical potassium channel along TAL (27), was exclusive to medullary TAL-α and TAL-γ cells (Figure 5A and Supplemental Figure 5F). A summary of the top 10 DEGs for each TAL cell type is presented in Supplemental Figure 5G, with a comprehensive list of DEGs included in Supplemental Data 1 and 2. Additional highlighted genes are presented in Supplemental Figure 6.
 Figure 5
Figure 5Regional expression of key proteins in TAL cell types and comparative analysis of TAL diversity: enriched-TAL versus whole-kidney TAL subset in RNA-Seq datasets. Heatmap showing regional gene expression patterns in TAL cell types. Pth1r and Casr transcripts are highest in TAL-β cells, peaking in the cortex. Avpr2 and Kcnt1 are enriched in claudin 10–positive TAL-α and TAL-γ cells, with highest levels in the medulla. Slc9a2 (NHE2) is primarily expressed in the cortex (TAL-α and TAL-β), while Slc9a3 (NHE3) is medulla enriched, peaking in TAL-γ cells. Data normalization and scaling: Gene expression data were normalized and scaled using a z-score to compare relative expression levels across clusters. “Avg Exp” indicates the z-scored mean gene expression within a cluster. (B–D) Comparative analysis of the enriched-TAL dataset with Slc12a1-positive TAL subsets extracted from a previously published whole-kidney RNA-Seq dataset (16). (B) UMAP comparing TAL cell types. The enriched-TAL dataset reveals 3 distinct TAL cell types: TAL-α, TAL-β, and TAL-γ, alongside MD and proliferating (Prolif) cells. In contrast, the whole-kidney TAL subset (16) predominantly identifies 2 TAL cell types (TAL-α and TAL-β) and MD cells. (C) UMAP illustrating the origin of TAL cells by kidney zone according to authors. In the enriched-TAL dataset, cells are derived from dissected cortex and medulla. In the whole-kidney dataset, kidney zones are designated as Zone 1 (cortex), Zone 2 (outer medulla), and Zone 3 (inner medulla) (16). (D) UMAP displaying Kcnj10 (Kir4.1) expression in both the enriched-TAL dataset and the whole-kidney TAL subset (16).
Regional expression patterns and functional insights. Our combined molecular and hand dissection approaches provided insight into regional gene expression patterns. Figure 5A compares relative expression across cell types and regions. Pth1r and Casr transcripts were most abundant in TAL-β cells, with peak expression in the cortex. Interestingly, a subset of cortical claudin 10–positive cells coexpressed Pth1r and Casr. In contrast, Avpr2 and Kcnt1 transcripts were enriched in claudin 10–positive TAL-α and TAL-γ cells, with highest levels in the medullary regions. Consistent with prior studies (14), Slc9a2 (NHE2) was predominantly expressed in the cortex, with similar levels in TAL-α and TAL-β cells, whereas Slc9a3 (NHE3) was enriched in the medulla, showing peak expression in TAL-γ cells.
As many published datasets do not contain anatomical information, we used the top 10 DEGs from cortical and medullary TAL cells to generate a cortex score and a medullary score (Supplemental Figure 7), serving as a robust spatial marker for TAL regions. Additional relevant DEGs are provided in Supplemental Figure 7.
TAL cell types and anatomical location are conserved across species. Immunolocalization results indicated similar ROMK expression heterogeneity in mouse, rat, and human (Supplemental Figure 1). To extend this, we examined TAL cell types across species, using published datasets from mouse, human, and rat kidneys (Supplemental Figures 8–10). The enhanced resolution obtained using the INTACT method was clearly evident when we compared our data with data from another mouse dataset (16) (Figure 5, B–D). There, we identified TAL-α cells, characterized by high Cldn10 expression, and TAL-β, marked by high Cldn16 expression (Supplemental Figure 8B), but TAL-γ cells did not form a distinct cluster (Supplemental Figure 8, C–E).
Analysis of the Kidney Precision Medicine Project (KPMP) human dataset (28) also revealed TAL-α and TAL-β clusters (Supplemental Figure 9, A–D). As with other datasets lacking TAL enrichment, TAL-γ cells were rare. Nevertheless, there was a strong and significant correlation between mouse and human TAL-α and TAL-β DEGs (Supplemental Figure 9, H and I).
In rats, unsupervised clustering of TAL cells also revealed clusters based on claudin 10 and claudin 16 expression. In this dataset, TAL-α cells further segregated into 2 subtypes (Supplemental Figure 10, A–D), likely from distinct kidney regions, but these cells could not be identified as TAL-γ. Regional markers (Clcnkb, Enox1 for the cortex; Clcnka, Slc4a7, Ank2, Cryab for the medulla) verified these spatial distinctions (Supplemental Figure 10E).
Across species, we observed that claudin 10–positive cells (TAL-α cells) consistently expressed key factors involved in sodium transport, as well as arachidonic acid signaling genes (Supplemental Figure 8F, Supplemental Figure 9E, and Supplemental Figure 10G) whereas claudin 16–positive (TAL-β) cells showed higher expression of genes associated with calcium and magnesium homeostasis (Supplemental Figure 8G, Supplemental Figure 9F, and Supplemental Figure 10H). DEG profiles of TAL-α and TAL-β cells were highly correlated between species (Supplemental Figures 9, H and I, and Supplemental Figure 10, J and K). A comprehensive list of DEGs for TAL-α and TAL-β cells across species is provided in Supplemental Data 1 and 2.
Furthermore, sex-based differences in TAL cell proportions were observed, with females showing a higher percentage of TAL-β cells in both mice and humans (Supplemental Figure 8J and Supplemental Figure 9J).
Ultrastructural features of TAL. The results described above indicate the existence of 3 cell types along the TAL, differing importantly in the expression of K+ channels. Some have speculated that differences in TAL conductance properties correspond to the appearance of “rough” (R) and “smooth” (S) TAL cells (11, 29). To examine this, we studied the morphology of medullary and cortical TAL epithelia under basal conditions using transmission (TEM) and scanning electron microscopy (SEM) in rat and mouse. In rat, TEM of ISOM showed that the luminal cell aspect of mTAL cells was a smooth plateau with few scattered, stubby microvilli, whereas cell borders were densely packed with marginal microvilli. Toward outer stripe and cortex, meandering of the TAL cell borders and density of marginal microvilli increased progressively along with the number of microvilli of the luminal plateau (Figure 6, A–I). Cells characteristically revealed a subapical vesicular compartment throughout with tightly packed vesicles. Subapical vesicles were most abundant in ISOM, less so in OSOM, and scarce in cTAL (Figure 6, D–F), verifying earlier data (30). Despite clearly visible axial heterogeneity of TAL epithelia in the respective kidney parenchymal zones, no evidence for luminal cell surface heterogeneity was detected within a given zone; this was verified by SEM showing no mosaic distribution of S or R cells in contrast with earlier descriptions (11, 31) (Figure 6, J–L). Similar findings were obtained in mouse kidney (Figure 6M).
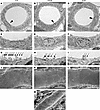 Figure 6
Figure 6Ultrastructural landmarks in TAL of rat and mouse kidney. TAL from cortex (CTX), OSOM, and ISOM. (A–F) Cross-sectional profiles (A–C) with increasing cell height toward ISOM; arrowheads indicate sites of higher magnification (D–F) by transmission electron microscopy. Note minor differences in microvillar density along the course of TAL. (G and H) Tight junctional fields show numbers of luminal microvilli along luminal cell borders decreasing from CTX to ISOM (arrows). (J–M) Scanning electron micrographs showing luminal cell aspects of TAL epithelium in rat (J–L) and mouse kidney (M). Note decreasing complexity of cell borders and numbers of microvilli in rat from CTX to ISOM as well as general absence of cell heterogeneity. Scale bars indicated.
Summary of results. Results have been schematized with respect to cell type characterization (Figure 7 and Supplemental Figure 12).
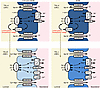 Figure 7
Figure 7Schematic drawing on cell type heterogeneity along TAL. Mechanistic model of TAL cellular heterogeneity. All cells express luminal NKCC2, basolateral chloride channels, and Na+/K+-ATPase to reabsorb NaCl via the transcellular path. TAL-α cells of all zones are equipped for paracellular Na+/K+ absorption via CLDN10. They express luminal ROMK and show low basolateral K+ conductance (LBC) to generate a lumen-positive voltage. TAL-β cells of CTX and OSOM comprise the equipment for paracellular Ca2+/Mg2+ reabsorption via CLDN16/19. They lack luminal ROMK and show high basolateral K+ conductance (HBC). TAL-γ cells of ISOM are equipped for paracellular Na+ absorption via CLDN10; they lack luminal ROMK and show HBC. TAL-β and TAL-γ cells per se do not contribute to transepithelial voltage, but adjacent LBC type TAL-α cells still provide the electrotonic driving force (dotted arrow) to support K+ reabsorption and paracellular passage of Na+ (TAL-α and TAL-γ cells) or Ca2+/Mg2+ (TAL-β cells). Data are from previous work (10–14, 32, 37) and current results.
-
Discussion
The TAL reabsorbs substantial amounts of filtered sodium, chloride, potassium, calcium, and magnesium and plays a central role in urinary concentration. It must be able to modulate ion transport selectively to meet homeostatic needs. Axial heterogeneity along the TAL is well recognized, but nearly all TAL cell models suggest a single TAL cell type, albeit some with variants (32). The present results show (Figure 7) that the mammalian TAL comprises 3 distinct cell types in addition to MD cells; each cell type expresses specific transport-related genes and regulatory proteins, suggesting that each responds to different stimuli and plays a unique role in homeostasis. Combining immunolocalization, enriched snRNA-Seq, and functional analyses, we now provide a model for solute transport pathways along the TAL that accounts for several previously confusing findings.
The results show that TAL cells express either apical ROMK or basolateral Kir4.1, not both, and that cells lacking apical ROMK have higher total K+ conductance. These results are consistent with early work showing that hamster TAL cells and amphiuma early distal tubule (its diluting segment) cells exhibit 2 patterns of membrane conductance, noted as HBC and LBC (10, 11). Additionally, the results show that cells with apical ROMK (TAL-α cells) express the monovalent cation-permeable claudin 10 at their tight junction, allowing them to both generate a transepithelial voltage and transport Na+ efficiently both through and around cells. As they also express NKCC2 at the apical membrane, TAL-α cells correspond to the traditional model for TAL cells in which nearly all K+ that enters across the apical membrane is recycled (33, 34). As might be expected, these claudin 10–expressing cells differentially express genes related to Na+ transport and water balance, such as Stk39 (encoding SPAK) (35), Avpr2 (encoding the vasopressin receptor), and Ptger3 (encoding prostaglandin E3 receptor) (36).
In contrast, cells in the cortex and OSOM that express Kir4.1 at their basolateral membrane also have NKCC2 at the apical membrane but do not exhibit apical ROMK; these cells express claudin 16 (TAL-β cells), marking them as Ca2+- and Mg2+-transporting cells. As might be expected, these cells differentially express genes encoding the PTH and vitamin D receptors. The absence of apical ROMK means that this cell type (TAL-β cells) does not contribute directly to transepithelial voltage generation. Yet, as Ca2+ and Mg2+ are reabsorbed via divalent ion-permeable claudin 16 (and 19), transport must be driven by the voltage generated by adjacent TAL-α cells. Additionally, in the cortex, where substantial divalent ion reabsorption occurs, the transepithelial voltage is increased further, owing to a Na+ diffusion potential across Na+-selective TAL-α cell tight junctions, reaching as high as 30 mV (32). In the ISOM, there is a third cell type that, like TAL-β cells, expresses Kir4.1 at the basolateral membrane and lacks ROMK at the apical membrane. These cells, however, have claudin 10 rather than claudin 16 within their tight junctions. We have labeled these TAL-γ cells, as they differ from the others, but these cells are often missed in analyses that do not enrich for TAL cells.
A key current finding is that ROMK/Kir4.1 mosaicism is present all along the TAL. Ultrastructural analysis clearly identified the onset of the ROMK signal at the tight junctions between ROMK-negative and ROMK-positive cells, validating an earlier suggestion that this is not an artifact (12, 13). Our electrophysiological findings in mice align with the transcriptomic and immunolocalization results, thereby extending prior transcriptomic studies in other species that often suggested 2 functional cell types, identified by differential claudin expression (10, 11). By integrating highly enriched transcriptomic analysis with immunolocalization and electrophysiology, and focusing on TAL cells across all kidney regions, a clear cell type differentiation appears. Wang and colleagues identified Kir4.1 (with Kir5.1) as responsible for K+-conductive pathways at the basolateral membrane of cTAL cells. They reported that 40 pS K+ channels were absent in the basolateral membrane of TAL cells from mice lacking Kcnj10 (Kir4.1), suggesting their identity (21). Interestingly, in light of the current results, they noted that Kir4.1 staining was “not uniform” along the TAL.
In terms of apical K+ conductance, we used the ROMK blocker TPNQ and the nonspecific K+ channel blocker Ba2+ and found that only a fraction of TAL cells exhibit ROMK activity (TPNQ sensitivity), consistent with our immunocytochemical results. These experiments also indicate that cells lacking ROMK exhibit higher whole-cell Ba2+-sensitive K+ conductance, again aligning with the mosaicism of K+ channels described (10, 11). Further, in perfused tubules, where we could control apical and basolateral composition selectively, we found that only a fraction of cells respond to changes in basolateral [K+]. Showing that this basolateral conductance is provided by cell-specific expression of Kir4.1 will require additional studies.
The loop of Henle is known to reabsorb K+, but mechanisms have been unclear. Although some K+ is believed to traverse the paracellular pathway driven by the transepithelial voltage, transcellular absorption also occurs (37). The reciprocity of K+ channels among different cell types is likely essential for TAL K+ reabsorption. TAL models throughout the years show that nearly all the K+ traversing the apical membrane via NKCC2 recycles back into the lumen (38, 39). Thus, owing to their high apical and low basolateral conductance, TAL-α cells should not mediate transcellular K+ reabsorption. Our data argue that transcellular K+ reabsorption is mediated by TAL-β and TAL-γ cells, which have NKCC2, lack an apical K+ exit pathway, and have high basolateral K+ conductance. Therefore, as shown in Figure 7, K+ entering TAL-β and TAL-γ cells must leave via the basolateral membrane, leading to net K+ reabsorption. The distinction between K+-reabsorbing cells (TAL-β and TAL-γ) and K+-recycling cells (TAL-α) may account for the observation that 30% of NaCl reabsorption remains in mice with ROMK deleted (40).
Although there is clear mosaicism regarding cells that express ROMK at the apical membrane and those that do not, all TAL cells exhibit message for Kcnj1 (ROMK), as documented through both snRNA-Seq and in situ hybridization, though Kcnj1 transcripts are more abundant in TAL-α cells. This suggests that there is a pool of Kcnj1 as a functional reservoir to adapt to altered reabsorptive workload or potassium homeostasis (41, 42). There is convincing evidence that diluting segments adjust K+ transport to meet homeostatic needs. In the amphiuma, dietary potassium adaptation increases the number of LBC cells (10), which according to our findings, express apical ROMK. Guggino and colleagues found that K+ loading changed the ratio of HBC to LBC cells (10) and suggested that K+ adaptation along this segment is mediated by cell type conversion rather than cell activation. Imai and colleagues reported that the ratio of LBC to HBC in hamster was greater in the cortical TAL, where net K+ secretion, rather than reabsorption, occurs (11). Finally, Sansom and colleagues reported that the loop of Henle of mice converts from a net potassium-reabsorbing segment to one that secretes potassium when animals consume a low-sodium/high-potassium diet (43). As both TAL-β and TAL-γ cells lack ROMK at baseline, it will be interesting to determine whether both cell types contribute to potassium adaptation.
The current results have implications with regard to diuretic effects and side effects. Furosemide is known as a “potassium-wasting” diuretic (44), with hypokalemia listed as a frequent complication of its use when treating edematous conditions. Sansom and colleagues (43), however, showed that furosemide became “potassium-sparing” when administered to mice receiving a diet that was high in potassium and low in sodium. As this effect persisted when amiloride was administered to block distal K+ secretion, they inferred that this resulted from effects along the TAL itself. Their cell model showed only a single type of TAL cell. Thus, they invoked a mechanism involving activation of NKCC2 and ROMK. Our results suggest an alternative or ancillary mechanism. If K+ loading alters the ratio of K+-reabsorbing cells to K+-secreting cells (10, 45), and if K+ absorption by both cell types is mediated by NKCC2, which can be inhibited by furosemide (see Figure 7), then the effects of the diuretic drug will depend on the ratio of cell types along this segment.
The present work extends recent transcriptomic analyses of human biopsies (46) and dissected mouse cortex (18), which typically identify 2 TAL clusters distinguished by the expression of CLDN10 and CLDN16. The nomenclature used for TAL clusters and their gene signatures, however, has not been consistent (17, 18, 20, 46). Our current results linking the K+ channel mosaicism with claudin expression, therefore, allow us to propose a cell type classification that provides substantial explanatory power. For example, although early work indicated that CaSR activation causes a diuresis (47) by inhibiting ROMK (48), more recent studies using highly selective CaSR agonists and antagonists show that activation or inhibition of the CaSR along the TAL can alter Ca2+ reabsorption independent of changes in transepithelial voltage (6, 8). This conclusion was confirmed recently in a tubule-specific CaSR-knockout mouse (49). At least part of this effect involves regulation of claudin 14, which interacts with claudins 16 and 19, thereby reducing Ca2+ permeability (50). Our results show that genes for the CaSR, the PTH receptor, and the vitamin D receptor are differentially expressed in TAL-β cells. The fact that we did not see CLDN14 likely reflects its limited expression in normocalcemic mice (51).
Our results further support the dominance of the circumferential CLDN16-positive tight junctional belt in TAL-β HBC cells, as they were arranged singly or in patches. While TAL-α cells expressed CLDN10 when they located next to each other, CLDN16 was observed where TAL-α and TAL-β cells were adjacent (Figure 3). Dominance is reflected by the share of cortical CLDN16 in total tight junctional length, which ranged between 37% and 97% in isolated tubules, suggesting its pronounced role in cTAL (15). The molecular cause for CLDN16 dominance remains unexplained, but it is also reflected in the widespread expression of CLDN16 at all cell borders when CLDN10 is deleted in mice (52) and in human HELIX syndrome, in which mutations in CLDN10 lead to hypermagnesemia and nephrocalcinosis (34).
A small snRNA-Seq cluster was identified here as well as in previous work (16, 17, 28, 46, 53) that expressed high levels of Nos1, identifying it as MD. Immunoreactivity for CLDN10 dominated in MD in agreement with earlier findings (23), and apical ROMK was mostly positive, but variable in strength, as was another marker of MD, cyclooxygenase-2 (54). Thus, along with CLDN16 absence, it may be concluded that the MD cell phenotype shares TAL-α properties (23).
Although it has been suggested that ROMK-positive cells correspond to microvilli-enriched or R as opposed to S cells with few microvilli (11), we could not confirm that ROMK expression was linked to structurally distinct R or S cells. Although we could reproduce the heterogeneity in axial cell structure, we failed to confirm mosaic arrangement of S and R cells intrasegmentally, i.e., in any given region of interest within mTAL or cTAL (55).
In conclusion, we show that there are 3 distinct cell types in TAL (TAL-α, TAL-β, and TAL-γ) arranged in a mosaic pattern across the TAL and conserved across species. Anchored in both the mosaicism of K+ channel expression and claudin expression, we assign independent regulation of paracellular routes for Na+ or Ca2+/Mg2+ to specific luminal or basolateral membrane proteins involved in transepithelial transport. These results suggest that one population of cells recycles K+ across the apical membrane, generates the transepithelial voltage, and may mediate K+ secretion, whereas another population (TAL-β cells), with high basolateral K+ conductance and expressing CLDN16, transports Ca2+ and Mg2+. Interestingly, TAL-γ cells, only along the ISOM, have some characteristics of each of the other types and seem poised to mediate both transcellular NaCl and K+ reabsorption (Figure 7). Our findings therefore imply that a diversity of TAL cell types, a feature conserved across species, enables inter- and intrasegmental heterogeneity in TAL to provide homeostasis of mono- and divalent cation transport at steady state and when challenged.
-
Methods
Sex as a biological variable. Both sexes were used for these studies. The results describe those findings shared by sexes and those where there are differences.
Tissues. For animal studies, adult male C57BL/6 mice (6 weeks old, n = 5; Janvier Labs) and male Long Evans rats (6 weeks old, n = 5; Janvier Labs) were used.
During housing, animals were kept in a 12-hour light/12-hour dark cycle, with ad libitum access to drinking water and standard rodent chow (Altromin 1324). Animals were anesthetized with a mixture of ketamine (Pfizer) and xylazine (Rompun). After opening the abdominal cavity, kidneys were perfusion-fixed via the abdominal aorta, first with 3% hydroxyethyl starch in 0.1 M Na-cacodylate (Caco) for 20 to 30 seconds, then with 3% paraformaldehyde (PFA)/3% hydroxyethyl starch in Caco for 5 minutes. Kidneys were removed and incubated in 800 mOsmol/kg H2O sucrose in PBS for 18 hours, shock-frozen in liquid nitrogen–cooled isopentane and stored at –80°C, or postfixed in 3% PFA in PBS overnight, dehydrated via graded ethanol series to xylene, and embedded in paraffin.
Electron microscopy. For ultrastructural analysis, kidney tissue was postfixed overnight at room temperature in 1.5% glutaraldehyde/1.5% PFA containing 0.05% picric acid in Caco, then in 1% osmium tetroxide/0.8% potassium hexacyanoferrate in Caco for 1.5 hours at room temperature (RT) for TEM or in 1% aqueous osmium tetroxide for SEM. Tissues were then dehydrated and embedded in epoxy resin for semithin sectioning and light microscopy or ultrathin sectioning and TEM analysis using standard methodology. For SEM, samples were high pressure-critical point-dried and sputter-coated. For pre-embedding immunoperoxidase staining 30 μm–thick cryostat sections were cut from frozen kidneys, stained with anti-ROMK antibody, and prepared for electron microscopic analysis as previously described (54).
Immunofluorescence. Cryostat kidney sections (6 μm thickness) were cut using a CM 3050S microtome (Leica) and permeabilized for 30 minutes with 0.5% Triton X-100 (Merck) in PBS. Paraffin sections (4 μm thickness) were prepared with an RM 2125 RT microtome (Leica), deparaffinized in xylene, and rehydrated via graded ethanol series. Heat-induced epitope retrieval was performed for 6 minutes in citrate buffer (pH 6.0) using a pressure cooker. Before antibody incubation, cryosections and paraffin sections were blocked with 5% skim milk (Difco, BD) for 30 minutes. Primary antibodies were then applied overnight at 4°C; after washing, secondary antibodies were applied for 60 minutes at RT (Supplemental Table 1). Nuclei were counterstained with DAPI (Sigma-Aldrich). Sections were mounted in 1:9 PBS/glycerol or ProLong Glass Antifade Mountant (Thermo Fisher Scientific) and examined using LSM 5 Exciter (ZEISS) or SP8 (Leica) confocal microscopes. The percentages of ROMK- and Kir4.1-negative cells were quantified in transversely cut TAL tubules and expressed as percentages of the total numbers of TAL cells counted per kidney zone. At least 20 similar tubular profiles were evaluated in each zone (inner stripe, outer stripe, cortex).
In situ hybridization. In situ hybridization was performed on perfusion-fixed, paraffin-embedded rat kidney tissue using an RNAscope 2.5 HD Brown Reagent Kit (Advanced Cell Diagnostics; ACD) according to manufacturer’s instructions. Briefly, dewaxed paraffin sections (4 μm thickness) were incubated with peroxidase blocker and boiled for 30 minutes at 100°C in a pretreatment solution. Sections were treated with protease for 30 minutes at 40°C. An ROMK target probe (ACD) was hybridized at 40°C for 2 hours in the dark using the HybEZ Hybridization System (ACD). After several amplifying and washing steps, the sections were stained with chromogenic substrate for detection of hybridization signals. ROMK mRNA signal was identified as red punctate dots. Nuclei were counterstained with hematoxylin for 2 minutes at RT. Sections were coverslipped with DakoCytomation Glycergel mounting medium. Sections were examined with a Leica DMRB microscope equipped with an AxioCam MRc camera (ZEISS). Endogenous housekeeping gene UBC was used as a positive control; bacterial dapB served as a negative control to assess background signal.
Electrophysiology. C57BL/6J mice (8 weeks old) were purchased from Jackson Laboratory. Mice were euthanized by CO2 inhalation plus cervical dislocation. Their abdomen was opened to expose the left kidney. Kidney was perfused with 2 mL L-15 medium (Life Technologies) containing type 2 collagenase (250 U/mL), then removed, and the cortex was dissected into small pieces for additional incubation in collagenase-containing L-15 media for 30–40 minutes at 37°C. Pieces were then washed 3 times with fresh L-15 medium and transferred to an ice-cold chamber for dissection. cTALs were isolated as described (21). Isolated cTALs were placed on a cover glass coated with poly-l-lysine. The cover glass was then transferred into a chamber mounted on an inverted microscope.
Patch-clamp experiments. We used an Axon Instruments AxoPatch 200A amplifier to conduct whole-cell voltage-clamp experiments in isolated and split-open cTAL. The pipette solution contained 140 mM KCl, 2 mM MgATP, 1 mM EGTA, and 10 mM HEPES (pH 7.4) for measurement of K+ currents. The bath solution was similar to the pipette solution without MgATP. The currents were low-pass–filtered at 1 kHz, then digitized by an Axon interface with a 4 kHz sampling rate (Digidata 1440A). Data were analyzed using pClamp software system 9.0 (Axon).
Membrane voltage measurement. C57BL/6 mice were euthanized, and the kidneys were removed and sliced for enzymatic tubule preparation as described (56). Tubule suspension was obtained by dissociation of slices in a thermomixer (Eppendorf, 850 rpm, 37°C) and mainly contained TAL from cortex and OSOM. The incubation solution was composed of (mM) 140 NaCl, 0.4 KH2PO4, 1.6 K2HPO4, 1 MgSO4, 10 Na-acetate, 1 α-ketoglutarate, and 1.3 calcium gluconate, pH 7.4, and contained (mg/L) 25 DNase I, 375 glycine, 48 trypsin inhibitor, and 2,000 collagenase II. For TAL sorting, the incubation solution was supplemented with 500 mg/L albumin. TALs were identified, isolated at 4°C according to morphological characteristics (56), and used for single-tubule immunofluorescence or membrane voltage measurements.
For immunofluorescence, single cTAL segments were placed on microscope slides (Epredia Polysine adhesion slides, Thermo Fisher Scientific) and fixed with 4% PFA for 7 minutes, washed several times with 10 mM sodium citrate pH 6, and heated in this buffer for 3 hours at 70°C. After washing with PBS-T (0.3% Triton X-100 in phosphate-buffered saline), tubules were incubated with primary antibodies (Supplemental Table 1) in PBS-T + 5% BSA overnight at 4°C. Secondary antibody (Supplemental Table 1) was incubated for 1–2 hours after extensive washing with PBS-T. Confocal images were acquired using ZEISS LSM 880 confocal laser-scanning microscope (excitation laser: 488, 594, and 633 nm) equipped with an Airyscan unit. Enzymatically dissected TALs were transferred into the bath chamber on the stage of a Stellaris 5 confocal microscope (Leica Microsystems) at 37°C and perfused by a concentric glass pipette perfusion system. Perfused TALs were stained by basolateral as well as luminal incubation with the membrane voltage dye Di-8-ANEPPS (6 μM; D3167, Thermo Fisher Scientific) in the presence of 0.02% Pluronic F-127 for 30 minutes in control solution containing (mM) 145 NaCl, 0.4 KH2PO4, 1.6 K2HPO4, 1 MgCl2, 1.3 calcium gluconate, and 5 glucose pH 7.4. After staining, fluorescence (excitation: 486 nm, emission: 505–700 nm) was recorded every 5 seconds at symmetric control solution. Then the tubule was superfused with 30 mmol/L K+ solution containing (mM) 120 NaCl, 26.4 KCl, 0.4 KH2PO4, 1.6 K2HPO4, 1 MgCl2, 1.3 calcium gluconate, and 5 glucose (pH 7.4) for 3 minutes. Fluorescence measurements were analyzed using LasX Office software (Leica Microsystems) after background subtraction. We selected 2 to 3 cells without movement artifacts, and fluorescence intensity was normalized to the mean intensity of 4 measurements preceding solution change. TAL cells were categorized into 2 groups, one with no increase in mean intensity in the presence of 30 K+ and another with mean intensity increase in the presence of 30 K+ upon 4 consecutive measurements.
Analysis of TAL snRNA-Seq dataset. NKCC2-Cre-INTACT mice in a C57BL/6 background were used for snRNA-Seq experiments. The Cre/LoxP system was used to label TAL nuclei with nuclear GFP, enabling fluorescence-activated nuclei sorting (FANS) enrichment. This approach, previously described for the DCT (25), uses the INTACT system to fluorescently label the nuclei of genetically targetable cell populations. The INTACT system tags the C-terminus of SUN1, a nuclear membrane protein, with 2 tandem copies of sfGFP and 6 copies of the Myc epitope (CAG-SUN1-sfGFP-Myc). To target TAL cells specifically, INTACT mice were crossed with NKCC2-CreERT2 mice (generated at University of South Florida), producing a mouse line that expresses sfGFP at the inner nuclear membrane in TAL cells upon tamoxifen induction.
To induce sfGFP expression, NKCC2-Cre-INTACT mice were intraperitoneally injected with 1 mg tamoxifen (dissolved in corn oil) daily for 5 days, followed by a 10-day induction period. Mice were housed under standard conditions with a 12-hour light/12-hour dark cycle and food and water ad libitum. After tamoxifen induction, mice were anesthetized using isoflurane, followed by cardiac perfusion with 10–15 mL of PBS to remove blood cells. Kidneys were harvested, and the kidney capsules were removed with the ureters trimmed prior to dissection. Prior to sequencing, kidneys were manually dissected into cortical and medullary regions. Kidneys were sliced along the cortical-medullary axis in a Petri dish. A superficial strip (~20% depth) from each slice was separated as the cortex zone, while the remaining tissue was collected as the medulla zone. The dissected cortex and medulla portions were snap-frozen in liquid nitrogen immediately after harvest and stored at –80°C until further downstream nuclei isolation, FANS, and snRNA-Seq.
Nuclei were isolated using the 10x Genomics kit (Chromium Nuclei Isolation Kit PN-1000494) according to the manufacturer’s instructions. The nuclei suspension was mixed with 5 μL Vybrant Ruby stain (Thermo Fisher Scientific) and incubated on ice for 15 minutes. The nuclei were sorted in 500 μL final resuspension buffer (FBS, 1 mL Dulbecco’s PBS with 1% BSA + 5 μL Protector RNase inhibitor, Roche) with a low flow rate and pressure to ensure high viability. Two main gates were used: a 561+683 emission for the ruby stain and a 488 530/40 emission for GFP. Low trigger pulse width was used as a singlet discriminator. One hundred fifty thousand nuclei were collected and centrifuged at 500g for 5 minutes at 4°C. The top supernatant was carefully removed, and 6 μL volume was left to resuspend the nuclei pellet.
snRNA-Seq was performed using a PIPseq T10 3′ Single Cell RNA Kit v5.0 (Fluent BioSciences). Single-nucleus suspensions with a maximal concentration of 3,400 nuclei/μL (for a total of 17,000 nuclei per sample) were added directly into Particle-templated Instant Partition (PIP) tubes. Following the manufacturer’s protocol, with subsequent vortexing the nuclei were partitioned into PIPs. Following capture and lysis of the RNA and downstream cDNA generation and amplification, the resulting cDNA was run on a 2% gel electrophoresis for approximate band size (greater than 500 bp). The cDNA was then used as a template for library preparation according to the reagent kit protocol (https://www.fluentbio.com/resource-category/user-guides/).
The raw sequencing data were aligned to GRCm39 (v. 2022) and deconvolved using PIPseeker 3.3.0 (Fluent BioSciences). The resulting dataset was processed using the Seurat pipeline, incorporating doublet removal with DoubletFinder, ambient RNA correction with SoupX, normalization via scTransform, and integration within Seurat. A 2D UMAP was generated using the first 10 principal components to visualize cellular clusters (27). Cortex and medulla scores were created using Seurat’s AddModuleScore function, based on the average expression of the top genes within cortex and medulla. Dimension, feature, dot, and violin plots were generated using Seurat’s built-in visualization functions.
The final enriched-TAL dataset included 31,884 features across 11,724 nuclei, comprising 4,076 TAL cortical nuclei with a median of 1,366 genes and a mean of 2,162 reads per nucleus, and 7,648 TAL medullary nuclei with a median of 1,412 genes and a mean of 2,320 reads per nucleus. A summary of PIPseeker outputs and the quality control of all nuclei is displayed in Supplemental Figure 11.
The previously published mouse dataset was downloaded from National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) (GSE129798) (16). Sample information was extracted from the data embedded into the cell barcodes. This dataset utilized whole-kidney single-cell suspensions from the cortex (Zone 1), outer medulla (Zone 2), and inner medulla (Zone 3) of 2 adult (8–9 weeks old) male and 2 adult female C57BL6/J mice. The human dataset from the KPMP was downloaded from 13 healthy references on November 29, 2022 (28). This dataset utilized single-nucleus suspensions from protocol renal biopsies, percutaneous nephrolithotomy biopsies, and living donor nephrectomy tissues. The samples contained spatial information, which KPMP determined by analyzing 5 μm histology sections adjacent to the sampled areas, reporting the relative composition of the cTAL and mTAL. The rat dataset was downloaded from GEO (GSE209821) (53). This dataset utilized whole-kidney single-nucleus suspensions from 3 male lean ZSF1 rats.
The whole-kidney datasets were analyzed using the Seurat Standard Workflow (Seurat v4.3.0) (57). For the mouse and rat datasets, the TAL cluster was identified by the abundant and specific expression of the TAL genetic marker Slc12a1. In the human dataset, the TAL was identified by subsetting cTAL, mTAL, and MD cells, following the original KPMP nomenclature. Erroneous cell clusters were removed prior to final reprocessing through the Seurat Standard Workflow. Profiles of Slc12a1-positive cells were analyzed using the Seurat R package through unsupervised clustering. Dimensionality reduction analysis was performed via principal component analysis. Clusters were generated by using Seurat’s built-in commands, FindNeighbors and FindClusters. The data were visualized as a 2D UMAP plot. Clusters were annotated based on prominent Cldn10, Cldn16, and Nos1 gene expression, as identified using the FindMarkers function. As a result, the clusters were named TAL-α, TAL-β, and MD cell types. The final Seurat object for the mouse, derived from 4 animals, comprised a total of 31,884 features across 1,529 nuclei. The final Seurat object for humans, derived from 13 individuals, comprised a total of 53,884 features across 9,410 nuclei. The final Seurat object for the rat, derived from 3 animals, comprised a total of 39,161 features across 8,408 nuclei. DEG analysis was conducted using Seurat’s FindMarkers function, ordering the resulting data frame according to the log2FC and an adjusted P value of 0.05. Plots were generated using Seurat’s built-in visualization functions.
Statistics. GraphPad Prism 8 software was used to analyze parameters. Quantification of cell counting data is presented as the mean ± SD. Evaluation of multiple groups used 1-way ANOVA followed by Tukey’s post hoc test. A P value less than 0.05 was considered significant.
Study approval. Animal studies in Germany were approved by the Berlin Senate (Berlin, Germany; animal experimental authorization G0285/10). Animal studies in Oregon were approved by the Oregon Health & Science University IACUC (protocol IP00286). Animal studies in New York were approved by New York Medical College (IACUC #21127). Human kidney samples were obtained from morphologically normal portions of tumor nephrectomy specimens after written consent (Charité Ethics Committee No. EA4/002/24).
Data availability. PIPseeker output files and an annotated Seurat object are uploaded to GEO (GSE284450). To ensure data accessibility, we also made the TAL-INTACT dataset available for further exploration via an interactive web tool generated using ShinyCell (58) at https://ellisonlab.shinyapps.io/tal_shinycell/Previously published data were used for this work (16, 28, 53). All study data are included in the article or supplement. Supporting raw data behind graphical analyses and means are provided in a supplemental file titled Supporting Data Values.
Code for data processing and figure generation is available on GitHub (https://github.com/JBLkidney/OHSU-NHT-Bahena_TAL_2024; commit ID e200a5a).
Prior publication: A preprint of this paper was published previously: Demirci H, et al. Distinct cell types along thick ascending limb express pathways for monovalent and divalent cation transport [preprint]. https://doi.org/10.1101/2025.01.16.633282 Posted on bioRxiv. January 21, 2025.
-
Author contributions
HD, JPBL, DHE, and SB designed research. HD, JPBL, AS, XTS, JWN, CLY, JNC, XPD, YS, DEY, CQ, NH, MB, WHW, and SB performed research. HD, JPBL, AS, XTS, JWN, CLY, JNC, XPD, YS, DEY, CQ, NH, MB, and SB analyzed data. KE and BE developed the PIPseq model. RL generated the NKCC2-CRE mouse model. HD, JPBL, DHE, and SB wrote the paper.
-
Acknowledgments
This work was financially supported by Deutsche Forschungsgemeinschaft BA700/22-2, MU2924/2-2, and SFB 1365-C04/-S01 to SB. It was also supported by the NIH R01 DK133220, DK51496, and U54TR001628 and a Fondation LeDucq: Transatlantic Network of Excellence 17CVD05. HD was supported by a doctoral fellowship from Ministry of National Education, Turkey. JPBL was supported by a Ben J. Lipps Research Fellowship from KidneyCure. JNC is supported by NIH T32HL094294. JWN is supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases DK121737.
We thank Kerim Mutig and Mingzhen Su for scientific advice, Anette Drobbe for secretarial help, Kerstin Riskowsky for expert technical help and Sara Timm, Petra Schrade, and John Horn (Core Facility for EM) for help with microscopy. We are grateful to Katharina Broeker for methodological help in RNAscope technology.
Address correspondence to: Sebastian Bachmann, Charité – Universitätsmedizin Berlin, Institut für Zell- und Neuroanatomie, Philippstr. 12, 10115 Berlin. Phone: 49.30.450.528.001; Email: sbachm@charite.de. Or to: David H. Ellison, Division of Nephrology and Hypertension (L334), Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Email: ellisond@ohsu.edu.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: JCI Insight. 2025;10(13):e190992. https://doi.org/10.1172/jci.insight.190992.
-
References
- Greger R. Coupled transport of Na+ and Cl– in the thick ascending limb of Henle’s loop of rabbit nephron. Scand Audiol Suppl. 1981;14 Suppl:1–15.View this article via: PubMed Google Scholar
- Greger R, Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflugers Arch. 1981;392(1):92–94.
- Burg MB, Green N. Function of the thick ascending limb of Henle’s loop. Am J Physiol. 1973;224(3):659–668.
- Meoli L, Gunzel D. The role of claudins in homeostasis. Nat Rev Nephrol. 2023;19(9):587–603.
- Plain A, et al. Corticomedullary difference in the effects of dietary Ca²+ on tight junction properties in thick ascending limbs of Henle’s loop. Pflugers Arch. 2016;468(2):293–303.
- Loupy A, et al. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest. 2012;122(9):3355–3367.
- Kleta R, Bockenhauer D. Salt-losing tubulopathies in children: what’s new, what’s controversial? J Am Soc Nephrol. 2018;29(3):727–739.
- Desfleurs E, et al. Calcium-sensing receptor: regulation of electrolyte transport in the thick ascending limb of Henle’s loop. Kidney Blood Press Res. 1998;21(6):401–412.
- Alexander RT, Dimke H. Effects of parathyroid hormone on renal tubular calcium and phosphate handling. Acta Physiol (Oxf). 2023;238(1):e13959.
- Guggino WB. Functional heterogeneity in the early distal tubule of the Amphiuma kidney: evidence for two modes of Cl– and K+ transport across the basolateral cell membrane. Am J Physiol. 1986;250(3 pt 2):F430–F440.View this article via: PubMed Google Scholar
- Tsuruoka S, et al. Axial heterogeneity of potassium transport across hamster thick ascending limb of Henle’s loop. Am J Physiol. 1994;267(1 pt 2):F121–F129.View this article via: PubMed Google Scholar
- Mennitt PA, et al. Localization of ROMK channels in the rat kidney. J Am Soc Nephrol. 1997;8(12):1823–1830.
- Xu JZ, et al. Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol. 1997;273(5):F739–F748.View this article via: PubMed Google Scholar
- Dimke H, Schnermann J. Axial and cellular heterogeneity in electrolyte transport pathways along the thick ascending limb. Acta Physiol (Oxf). 2018;223(1):e13057.
- Milatz S, et al. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc Natl Acad Sci U S A. 2017;114(2):E219–E227.
- Ransick A, et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell. 2019;51(3):399–413.e7.
- Muto Y, et al. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat Commun. 2021;12(1):2190.
- Chen L, et al. Targeted single-cell RNA-seq identifies minority cell types of kidney distal nephron. J Am Soc Nephrol. 2021;32(4):886–896.
- Kirita Y, et al. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A. 2020;117(27):15874–15883.
- Wilson PC, Muto Y, Wu H, Karihaloo A, Waikar SS, Humphreys BD. Multimodal single cell sequencing implicates chromatin accessibility and genetic background in diabetic kidney disease progression. Nat Commun. 2022;13(1):5253.
- Zhang C, et al. KCNJ10 (Kir4.1) is expressed in the basolateral membrane of the cortical thick ascending limb. Am J Physiol Renal Physiol. 2015;308(11):F1288–F1296.View this article via: CrossRef Google Scholar
- Jin W, Lu Z. Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1999;38(43):14286–14293.
- Prot-Bertoye C, et al. Differential localization patterns of claudin 10, 16, and 19 in human, mouse, and rat renal tubular epithelia. Am J Physiol Renal Physiol. 2021;321(2):F207–F224.
- Quintanova C, et al. Unrecognized role of claudin-10b in basolateral membrane infoldings of the thick ascending limb. Ann N Y Acad Sci. 2022;1517(1):266–278.
- Su XT, et al. Enriched single-nucleus RNA-sequencing reveals unique attributes of distal convoluted tubule cells. J Am Soc Nephrol. 2024;35(4):426–440.
- Huber RD, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol. 2007;292(2):C795–C806.
- de Combiens E, et al. The apical 70-pS potassium K channel in the thick ascending limb of Henle’s loop is a large-conductance Na(+)-and Cl(-)-activated, K(Na)1.1-like, channel. Am J Physiol Renal Physiol. 2024;326(3):F485–F496.View this article via: CrossRef Google Scholar
- Lake BB, et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature. 2023;619(7970):585–594.
- Mount DB. Thick ascending limb of the loop of Henle. Clin J Am Soc Nephrol. 2014;9(11):1974–1986.
- Bachmann S, et al. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry. 1985;83(6):531–538.
- Nielsen S, et al. Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol. 1998;275(6):F885–F893.View this article via: PubMed Google Scholar
- Bankir L, et al. Medullary and cortical thick ascending limb: similarities and differences. Am J Physiol Renal Physiol. 2020;318(2):F422–F442.
- Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol. 1984;246(6 pt 2):F745–F756.View this article via: PubMed Google Scholar
- Vargas-Poussou R. Pathophysiological aspects of the thick ascending limb and novel genetic defects: HELIX syndrome and transient antenatal Bartter syndrome. Pediatr Nephrol. 2022;37(2):239–252.
- Richardson C, et al. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci. 2011;124(pt 5):789–800.
- Jensen BL, et al. Differential regulation of renal prostaglandin receptor mRNAs by dietary salt intake in the rat. Kidney Int. 1999;56(2):528–537.
- Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest. 1982;70(2):219–229.
- Greger R, Schlatter E. Properties of the basolateral membrane of the cortical thick ascending limb of Henle’s loop of rabbit kidney. A model for secondary active chloride transport. Pflugers Arch. 1983;396(4):325–334.
- Sohail S, et al. A brief history of the cortical thick ascending limb: a systems-biology perspective. Am J Physiol Renal Physiol. 2024;328(1):F82–F94.
- Bailey MA, et al. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter’s syndrome and in adaptation to a high-K diet. Kidney Int. 2006;70(1):51–59.
- Lu M, et al. ROMK is required for expression of the 70-pS K channel in the thick ascending limb. Am J Physiol Renal Physiol. 2004;286(3):F490–F495.
- Bankir L, et al. Adaptation of the rat kidney to altered water intake and urine concentration. Pflugers Arch. 1988;412(1–2):42–53.
- Wang B, et al. Net K+ secretion in the thick ascending limb of mice on a low-Na, high-K diet. Kidney Int. 2017;92(4):864–875.
- Novak JE, Ellison DH. Diuretics in States of Volume Overload: Core Curriculum 2022. Am J Kidney Dis. 2022;80(2):264–276.
- Yoshitomi K, et al. Functional heterogeneity in the hamster medullary thick ascending limb of Henle’s loop. Pflugers Arch. 1987;408(6):600–608.
- Li H, et al. Transcriptomic, epigenomic, and spatial metabolomic cell profiling redefines regional human kidney anatomy. Cell Metab. 2024;36(5):1105–1125.e10.
- Hebert SC. Calcium and salinity sensing by the thick ascending limb: a journey from mammals to fish and back again. Kidney Int Suppl. 2004(91):S28–S33.View this article via: PubMed Google Scholar
- Wang W, et al. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am J Physiol. 1997;273(3 pt 2):F421–F429.View this article via: PubMed Google Scholar
- Himmerkus N, et al. Calcium-sensing receptor in the thick ascending limb and renal response to hypercalcemia. J Am Soc Nephrol. 2025.
- Lee JJ, et al. Activation of the calcium sensing receptor increases claudin-14 expression via a PLC-p38-Sp1 pathway. FASEB J. 2021;35(11):e21982.
- Frische S, et al. Localization and regulation of claudin-14 in experimental models of hypercalcemia. Am J Physiol Renal Physiol. 2021;320(1):F74–F86.
- Breiderhoff T, et al. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A. 2012;109(35):14241–14246.
- Balzer MS, et al. Treatment effects of soluble guanylate cyclase modulation on diabetic kidney disease at single-cell resolution. Cell Rep Med. 2023;4(4):100992.
- Cåmpean V, et al. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am J Physiol Renal Physiol. 2003;285(1):F19–F32.
- Imai M, et al. Morphological and functional heterogeneity of the thick ascending limb of Henle’s loop. Clin Exp Nephrol. 1999;3:9–17.View this article via: CrossRef Google Scholar
- Gong Y, et al. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012;31(8):1999–2012.
- Hao Y, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–3587.e29.
- Ouyang JF, et al. ShinyCell: simple and sharable visualization of single-cell gene expression data. Bioinformatics. 2021;37(19):3374–3376.
- Greger R. Coupled transport of Na+ and Cl– in the thick ascending limb of Henle’s loop of rabbit nephron. Scand Audiol Suppl. 1981;14 Suppl:1–15.
-
Version history
- Version 1 (June 5, 2025): In-Press Preview
- Version 2 (July 8, 2025): Electronic publication



![Functional evidence for distinct cell types with or without ROMK activity that respond to basolateral [K+]. Functional evidence for distinct cell types with or without ROMK activity t](http://df6sxcketz7bb.cloudfront.net/manuscripts/190000/190992/small/jci.insight.190992.f2.gif)







