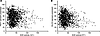Clinical Research and Public HealthPulmonologyTherapeutics Free access | 10.1172/jci.insight.126703
Angiotensin-converting enzyme inhibitors may affect pulmonary function in lymphangioleiomyomatosis
Wendy K. Steagall,1 Mario Stylianou,2 Gustavo Pacheco-Rodriguez,1 and Joel Moss1
1Pulmonary Branch and
2Office of Biostatistics Research, National Heart, Lung, and Blood Institute, NIH, Bethesda, Maryland, USA.
Address correspondence to: Joel Moss, Room 6D05, Building 10, MSC 1590, National Institutes of Health, Bethesda, Maryland 20892-1590, USA. Phone: 301.496.1597; Email: mossj@nhlbi.nih.gov.
Find articles by Steagall, W. in: PubMed | Google Scholar
1Pulmonary Branch and
2Office of Biostatistics Research, National Heart, Lung, and Blood Institute, NIH, Bethesda, Maryland, USA.
Address correspondence to: Joel Moss, Room 6D05, Building 10, MSC 1590, National Institutes of Health, Bethesda, Maryland 20892-1590, USA. Phone: 301.496.1597; Email: mossj@nhlbi.nih.gov.
Find articles by Stylianou, M. in: PubMed | Google Scholar
1Pulmonary Branch and
2Office of Biostatistics Research, National Heart, Lung, and Blood Institute, NIH, Bethesda, Maryland, USA.
Address correspondence to: Joel Moss, Room 6D05, Building 10, MSC 1590, National Institutes of Health, Bethesda, Maryland 20892-1590, USA. Phone: 301.496.1597; Email: mossj@nhlbi.nih.gov.
Find articles by Pacheco-Rodriguez, G. in: PubMed | Google Scholar
1Pulmonary Branch and
2Office of Biostatistics Research, National Heart, Lung, and Blood Institute, NIH, Bethesda, Maryland, USA.
Address correspondence to: Joel Moss, Room 6D05, Building 10, MSC 1590, National Institutes of Health, Bethesda, Maryland 20892-1590, USA. Phone: 301.496.1597; Email: mossj@nhlbi.nih.gov.
Find articles by Moss, J. in: PubMed | Google Scholar
Published March 7, 2019 - More info
JCI Insight. 2019;4(5):e126703. https://doi.org/10.1172/jci.insight.126703.
© 2019 American Society for Clinical Investigation
Received: December 13, 2018; Accepted: January 25, 2019
-
Abstract
INTRODUCTION. A local renin-angiotensin system exists in the pulmonary nodules of lymphangioleiomyomatosis patients. Sirolimus, the standard treatment for lymphangioleiomyomatosis, stabilizes lung function, but all patients do not respond to or tolerate sirolimus. As renin-angiotensin systems may affect tumor growth and metastasis, we questioned if angiotensin-converting enzyme inhibitors affected lymphangioleiomyomatosis disease progression.
METHODS. Retrospective study of 426 patients was performed, examining angiotensin-converting enzyme levels, pulmonary function data, and angiotensin-converting enzyme inhibitor treatment.
RESULTS. Serum angiotensin-converting enzyme levels were elevated in approximately 33% of patients, increased with duration of disease, and were inversely correlated with pulmonary function. Levels decreased significantly over time with sirolimus treatment. Treatment with angiotensin-converting enzyme inhibitors was reported by approximately 15% of patients and was significantly associated with a slower rate of decline in percentage predicted forced expiratory volume (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO) in patients not treated with sirolimus. No significant differences in rates of decline of FEV1 or DLCO were seen in patients treated with both inhibitors and sirolimus versus sirolimus alone.
CONCLUSIONS. Angiotensin-converting enzyme inhibitors may slow decline of pulmonary function in patients with lymphangioleiomyomatosis not treated with sirolimus. These inhibitors may be an option or adjunct in the treatment of lymphangioleiomyomatosis. A clinical trial may be warranted to examine this possibility.
FUNDING. NIH.
-
Introduction
The classical renin-angiotensin system (RAS) is associated with systemic arterial pressure regulation (1). Renin, secreted from the kidney into the bloodstream, cleaves angiotensinogen (AGT) (expressed by the liver) to produce angiotensin I, which is further cleaved by angiotensin-converting enzyme (ACE) (expressed by the lungs), generating angiotensin II (AngII). AngII, working through the AngII type 1 receptor (AT1R), regulates blood pressure and maintains fluid and electrolyte homeostasis. A local RAS is present in most tissues and contributes to the growth and differentiation of mesenchymal cell types (2). RAS dysfunction is implicated in bronchial hyperresponsiveness, airway remodeling, pulmonary hypertension, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome, and asthma (3). RAS components are also present in different cancer types and may enhance survival, proliferation, and metastasis of tumor cells (4, 5). Treatment with ACE inhibitors (ACEIs) is associated with a decreased risk of developing certain cancers (6, 7), and slows pulmonary function decline in patients with pulmonary diseases (8).
Risk of developing cancer is significantly lower in patients receiving ACEIs compared with controls or to patients receiving other antihypertensive drugs (6, 7). A meta-analysis of 55 studies examining the relationship between overall survival and RAS inhibition concluded that significant improvement in survival was seen in RAS-inhibitor-treated patients with renal cell carcinoma, gastric cancer, pancreatic cancer, hepatocellular carcinoma, upper-tract urothelial carcinoma, and bladder cancer compared with patients without RAS inhibitor treatment (7). There was also a trend toward better outcome for patients with lung cancer and melanoma treated with RAS inhibitors.
Lymphangioleiomyomatosis (LAM) is a metastatic multisystem disease primarily affecting women, with manifestations in lungs, kidneys (angiomyolipomas [AMLs]), and/or lymphatics (lymphangioleiomyomas [LLMs]) (9, 10). LAM is characterized by abnormal smooth muscle–like LAM cell proliferation and may occur sporadically or as a component of tuberous sclerosis complex (TSC), an autosomal dominant disease affecting the brain, lung, kidneys, heart, and skin. LAM lungs are characterized by cysts surrounded by LAM nodules, composed of LAM cells, type II pneumocytes, lymphatic endothelial cells, fibroblasts, lymphocytes, and mast cells (11–14). LAM cells have mutations in the TSC2 gene (15), resulting in dysfunctional tuberin that, with hamartin (TSC1), forms a complex controlling the mechanistic target of rapamycin (mTOR) (16). Loss of TSC2 leads to mTOR hyperactivity, resulting in abnormal cell growth and proliferation (16). The mTOR inhibitor, sirolimus (rapamycin), stabilizes pulmonary function and decreases the size of AMLs, LLMs, and chylous effusions (17, 18), although not all patients respond to or tolerate sirolimus (19, 20).
We have demonstrated the presence of LAM-specific RAS, with the identification of AGT, AngII, ACE, renin, chymase, AT1R, and AT2R in LAM lung nodules (12). In 2 studies with a limited number of LAM patients, serum ACE activity was significantly elevated compared with healthy volunteers, but did not correlate with pulmonary function (21, 22), nor did ACE activity decrease with sirolimus treatment (22). To test our hypotheses that ACE has a role in LAM disease and that ACEIs may slow disease progression, we examined serum ACE activity levels and the effect of ACEIs on pulmonary function of 426 LAM patients. Serum ACE levels correlated inversely with pulmonary function, and treatment with ACEIs resulted in slower rates of decline in pulmonary function. A prospective clinical trial examining the utility of ACE inhibition for the LAM population may be warranted.
-
Results
ACE activity is elevated in a third of LAM patients. We measure ACE activity levels routinely in serum of LAM patients; an upper level of 52 U/l was determined by the NIH Clinical Center after analyzing the ACE activity levels of 28 healthy fasting normal volunteers. Three hundred sixteen patients had fasting ACE activity measurements (6.1 ± 0.2 measurements per patient) performed while not receiving sirolimus therapy (either never on treatment or pretreatment) or ACEIs. One hundred five patients had at least one measurement greater than 52 U/l, resulting in 33.2% of patients with a higher than normal ACE activity level (Figure 1). Seventy-two (22.8%) patients had higher than normal ACE activity levels measured on at least 50% of visits (Figure 1, purple box), while 39 (12.3%) had higher levels at all visits (Figure 1, red box).
 Figure 1
Figure 1One hundred five patients (not on sirolimus treatment, out of 316 patients examined) had at least one fasting serum ACE activity level greater than 52 U/l (the upper limit established by the NIH Clinical Research Center, black line). Each column of dots represents the ACE activity levels of a patient, measured at visits to the Clinical Center. Gray box: patients 1–33 had ACE activity levels greater than 52 U/l less than 50% of the time. Purple box: patients 34–66 had ACE activity levels greater than 52 U/l at least 50% of the time. Red box: patients 67–105 had ACE activity levels greater than 52 U/l at all visits.
ACE activity increases over time and decreases with sirolimus treatment. Statistical analysis was performed using mixed-effects models that account for multiple measurements of ACE activity for each patient. Fifty-nine patients had ACE activity measurements both before and during sirolimus treatment. In these patients, ACE activity increased over time before sirolimus (0.511 ± 0.262 U/l/year) and decreased during treatment (–1.527 ± 0.333 U/l/year) (P < 0.001) (Figure 2, A and B). A similar result was seen when the comparison was expanded to all patients not receiving sirolimus versus patients during treatment; ACE activity levels increased over time in patients not receiving sirolimus (0.966 ± 0.092 U/l/year) (Figure 2C) and decreased over time (–2.332 ± 0.275 U/l/year) in patients treated with sirolimus (P < 0.001) (Figure 2D). Therefore, ACE activity levels increase with disease progression and decrease with sirolimus treatment.
 Figure 2
Figure 2Serum ACE activity before and after sirolimus therapy. (A) Serum ACE activity increased over time in 59 patients before receiving sirolimus therapy (blue, 0.511 ± 0.262 U/l/year) (mean ± SEM) and decreased over time during sirolimus treatment (orange, –1.527 ± 0.333 U/l/year) (P < 0.001). (B) Representative plots of ACE activity over time following 25 patients before and during sirolimus treatment. (C) The rate of change of ACE activity was 0.966 ± 0.092 U/l/year in 316 patients either never on sirolimus or pretreatment. (D) The rate of change of ACE activity was –2.332 ± 0.275 U/l/year in 73 patients during sirolimus treatment. The rates of change are significantly different (P < 0.001). Statistical analysis was performed using mixed-effects models that account for multiple measurements of ACE activity for each patient and adjusting for the initial value and time of visit.
ACE activity correlates with VEGF-D. AngII, through AT1R, induces VEGF synthesis (5). VEGF-D is a biomarker for LAM, with levels greater than 800 pg/ml considered diagnostic for LAM in conjunction with characteristic cysts on high-resolution CT scans (9). We compared ACE levels to the available VEGF-D levels for 48 patients not receiving sirolimus or RAS inhibition and found that ACE activity was significantly correlated with VEGF-D (r = 0.639, P < 0.001) (Figure 3).
 Figure 3
Figure 3ACE activity correlated significantly (P < 0.001) with VEGF-D levels measured at the same visit. Serum VEGF-D levels were available for 48 patients not receiving sirolimus or ACEIs.
ACE activity is inversely associated with pulmonary function. ACE activity levels were inversely associated with percentage predicted forced expiratory volume (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO) (both P < 0.001) using mixed-effects models that adjust for the initial pulmonary function values and sirolimus treatment. Higher ACE activity was associated with lower percentage predicted FEV1 and DLCO (Figure 4). These data suggest that ACE is an important factor in LAM disease progression.
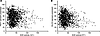 Figure 4
Figure 4Serum ACE activity was inversely correlated with FEV1 and DLCO. Serum ACE activity was inversely correlated with percentage predicted FEV1 (A) and DLCO (B) (both P < 0.001) using mixed-effects models controlling for initial values and sirolimus treatment. Measurements of ACE activity from 330 patients (including both measurements before and during sirolimus treatment; 257 patients never began sirolimus therapy, 59 patients have measurements both before and during therapy, and 14 patients have measurements only during therapy) were compared to the percentage predicted FEV1 or DLCO values recorded at the same visit to the NIH. The trend lines on the graphs were created by Excel and are for visual purposes only, as mathematical models were created for both percentage predicted FEV1 and DLCO. The models for patients not on sirolimus are (i) FEV1 = 18.4895 + (0.9156 × [initial FEV1]) – (0.06942 × [ACE activity]) – 10.4531 – (1.4638 × time); (ii) DLCO = 17.4347 + (0.9044 × [initial DLCO]) – (0.09381 × [ACE activity]) – 8.9540 – (1.4636 × time). During sirolimus therapy, the models are (iii) FEV1 = 18.4895 + (0.9156 × [initial FEV1]) – (0.06942 × [ACE activity]) – (1.4638 × time); (iv) DLCO = 17.4347 + (0.9044 × [initial DLCO]) – (0.09381 × [ACE activity]) – (1.4636 × time).
Treatment with ACEIs has a significant effect on the rate of decline of pulmonary function. The study population comprised 426 female patients (Table 1). Three hundred sixty patients were never treated with ACEIs, while 66 patients received ACEIs for 2.81 ± 0.33 years (Table 2). The effect of ACEIs on pulmonary function was analyzed using mixed-effects models that adjust for the initial pulmonary function values and sirolimus treatment. Patients receiving ACEI treatment had higher FEV1, forced vital capacity (FVC), FEV1/FVC, and DLCO than those not receiving ACEIs, but the differences were either not significant or only marginally significant (Table 3). However, the rates of decline of all pulmonary function measures (except for FEV1/FVC) were significantly slower in patients receiving ACEIs than those not treated with ACEIs (P ≤ 0.006) (Table 3).
Interestingly, analysis of the pulmonary function rates of change revealed a statistical interaction between the predictors “ACEIs” and “sirolimus treatment” (P < 0.004), indicating that the effect of the ACEI treatment differed for patients being treated with sirolimus versus those not treated with sirolimus. This prompted us to examine the no-sirolimus-treatment subgroup (406 patients) separately from the sirolimus-treatment subgroup (110 patients). The no-sirolimus-treatment subgroup includes patients never on sirolimus and those with pretreatment data (Table 1). Table 4 shows the rates of change of various pulmonary function values over time for each subgroup. The no-sirolimus-treatment subgroup has significantly slower rates of decline of pulmonary function (except FEV1/FVC) in the patients treated with ACEIs than in those not treated with ACEIs. The rates of change of most of the pulmonary function values did not vary significantly in the sirolimus-treatment subgroup between those receiving ACEIs and those not receiving ACEIs, except for percentage predicted FVC, which appears to have a slower rate of improvement in the presence of ACEIs and sirolimus versus sirolimus alone (P = 0.015).
 Table 4
Table 4Effect of ACEIs on the rate of change of pulmonary function in the no-sirolimus-treatment subgroup versus the sirolimus-treatment subgroup
-
Discussion
Serum ACE levels were elevated in approximately 33% of LAM patients for at least one visit and in 12.3% of patients for all visits (Figure 1), and higher serum ACE levels were inversely associated with lower FEV1 and DLCO (Figure 4). Serum ACE levels increased over time in patients not receiving sirolimus but decreased significantly in patients treated with sirolimus (Figure 2). These data suggest that ACE may have a role in LAM progression. Both a study with 58 LAM patients (45 with definite LAM, 13 with probable LAM) (21) and one with 102 LAM patients (conference abstract) (22) found serum ACE levels to be significantly higher in LAM patients than healthy controls. Neither study found an association with pulmonary function, nor did one study find an effect of sirolimus on serum ACE levels (22). These differences may be attributable in part to the number of patients examined; our study includes multiple serum ACE measurements for 330 LAM patients.
We examined the effects of ACE inhibition on pulmonary function of LAM patients through a retrospective study involving 426 patients. Treatment with ACEIs resulted in significantly slower rates of decline of pulmonary function than those seen with no ACEI treatment (Table 3) using mixed-effects models that adjusted for sirolimus treatment. When the group was divided into no-sirolimus-treatment versus sirolimus-treatment groups, treatment with ACEIs resulted in slower rates of decline in pulmonary function in patients not being treated with sirolimus; however, the rates were not significantly different in patients treated with sirolimus (Table 4), suggesting that sirolimus and ACEIs may impact the same pathway in LAM disease or that sirolimus treatment overwhelms ACEI treatment effects. AGT, AngII, ACE, renin, chymase, AT1R, and AT2R are present in pulmonary LAM nodules (12). Chymase may also cleave AngI to produce AngII (23) in LAM lung nodules. Activation of a LAM-specific RAS or dysregulation of the pulmonary RAS could promote LAM disease in several ways, including stimulation of angiogenesis/lymphangiogenesis, metastasis, inflammation, and cell proliferation, and effects on the LAM cell microenvironment and tissue remodeling (4, 5) (Figure 5). RAS signaling is complex; here, we will focus on possible effects due to stimulation of AT1R by AngII.
 Figure 5
Figure 5Pathways impacted by RAS that may play a role in LAM disease. The components of RAS found in LAM lung nodules include AGT, renin, ACE, AngII, AT1R, and AT2R, while chymase, which can also cleave AngI to generate AngII, is detected in mast cells present in the nodules as well (components of LAM RAS in red). Stimulation of AT1R by AngII can promote fibrogenesis and tissue remodeling (through TGF-β), cell proliferation and/or motility (through TGF-β, Stat3, PKC, tyrosine kinases [TyrK], serine/threonine kinases [Ser/Thr-K], and ERK and Akt inhibition of TSC2 resulting in mTOR activation), angiogenesis/lymphangiogenesis (through VEGF), and metastasis (due to VEGF-stimulated lymphangiogenesis and IL-6–, IL-8–, IL-1α–, IL-1β–, Cox2-, and MCP1-stimulated inflammation and creation of a protumorigenic environment). MMPs also affect tissue remodeling and are present in LAM lung nodules, with a sirolimus-insensitive increase in MMP2 expression in TSC2-deficient cells. Sirolimus inhibits mTOR activity and is the treatment of choice for LAM patients. ARBs block the activation of AT1Rs, while ACEIs inhibit ACE activity and the cleavage of AngI to AngII. ACEIs can directly bind to MMPs and inhibit their activity; thus, ACEIs may impact an extra aspect of tissue remodeling not affected by ARBs.
The RAS plays a role in tumor-associated angiogenesis, with induction of VEGF by AngII in tumor stroma (24). LAM is characterized by high levels of VEGF-D (25), which are associated with lymphangiogenesis (14). In a mouse model of gastric cancer, RAS inhibition significantly decreased lymphatic microvessel density and VEGF-C expression (26). VEGF-C and VEGF-D induce LAM cell proliferation through cross-talk with lymphatic endothelial cells (27). Here, ACE activity was significantly correlated with VEGF-D (Figure 3), suggesting that an increase in AngII generation by ACE may result in increased VEGF-D (5).
The RAS may affect the tumor microenvironment (Figure 5), as stromal cells such as endothelial cells, fibroblasts, monocytes, macrophages, and neutrophils may express components of RAS (28). Cancer-associated fibroblasts can be regulated by RAS to maintain inflammation and promote a protumorigenic microenvironment. Fibroblasts within the LAM nodule affect the LAM cell microenvironment and intercellular signaling (13). AngII can stimulate the release of cytokines from both tumor and stromal cells, including IL-6, IL-8, COX-2, MCP-1, and TGF-β (28). Expression of COX-2 is elevated in TSC2-deficient cells and LAM lungs, and through effects on prostaglandin production may impact cancer development (29). Similarly, MCP-1 levels in bronchoalveolar lavage fluid from LAM patients are significantly higher than those seen in female normal volunteers, and MCP-1 stimulates the migration of LAM cells (30).
Dysregulated TGF-β signaling plays a role in COPD, with elevated expression of TGF-β in the lungs (31). AngII stimulates TGF-β expression through AT1R (28). A mouse model of COPD, with cigarette smoke exposure, showed airspace destruction and increased TGF-β and lung size (31). Mice treated with losartan, an angiotensin II receptor blocker (ARB), showed normal lung size and lung elastance and reduced inflammatory cell infiltration into the lungs (31). Abundant TGF-β was observed by immunohistochemistry in LAM lungs (32), as was fibronectin, which is expressed in response to TGF-β, suggesting that fibronectin and TGF-β may stimulate LAM cell proliferation and lung remodeling (32). Inhibition of AngII activation of TGF-β expression by ARB or ACEI may impact lung remodeling in LAM.
RAS may affect cell signaling pathways known to have a role in LAM pathogenesis (Figure 5). Levels of p-Stat1/Stat1 and p-Stat3/Stat3 were increased in LAM lung compared with normal lung, suggesting a perturbation in Jak/Stat signaling in TSC-deficient cells (33). Stat3 activation is also required for proliferation and survival of TSC-dysfunctional cells (34). AngII induces the phosphorylation of both Stat1 and Stat3 in vascular smooth muscle cells through AT1R (35), and thus, may play a role in LAM cell proliferation and survival.
Stimulation of AT1R by AngII activates Akt and ERK pathways, leading to inhibition of TSC2 and activation of mTOR, resulting in cell growth and proliferation (36). Hyperactivation of mTOR due to the loss of TSC2 function is a characteristic of LAM, and sirolimus is administered to slow LAM cell growth. We found that the rates of decline in pulmonary function were not significantly different between patients treated with sirolimus and ACEIs and those treated with sirolimus alone (Table 4). This result suggests that sirolimus and ACEIs are both affecting the mTOR pathway, as the effects were not additive. Sirolimus may also be decreasing the number of LAM cells, leading to a decrease in ACE expression/activity, and resulting in less effect of ACEIs. While not statistically significant, treatment with ACEIs in addition to sirolimus may reduce the decline in pulmonary function (FEV1: 0.164% ± 0.431% predicted/year; DLCO: 0.132% ± 0.292% predicted/year) in comparison with the continued decline seen with sirolimus alone (FEV1: –0.135% ± 0.135% predicted/year; DLCO: –0.162% ± 0.092% predicted/year) (Table 4). It may be necessary to follow more patients for longer to determine if RAS inhibition, which might influence more aspects of LAM pathogenesis and progression than just mTOR, could significantly add to the sirolimus-induced effects on pulmonary function.
Matrix metalloproteinases (MMPs) participate in the cystic destruction of the LAM lung (37), especially MMP-2 and MMP-9. Expression of MMP-2 is upregulated in TSC2-deficient cells in a sirolimus-insensitive manner (37). ACEIs can inhibit MMP activity directly by binding to the active site of the MMP (38, 39). ACEI treatment may be affecting the LAM cell by inhibition of MMPs, thereby slowing the cystic destruction of the lungs, resulting in slower rates of decline in lung function over time.
The most frequent adverse reactions to sirolimus therapy (in at least 40% of patients) include hypercholesterolemia, upper respiratory tract infections, stomatitis, diarrhea, peripheral edema, acne, hypertension, headaches, and leukopenia (20). Some LAM patients are unable to continue treatment with sirolimus due to the side effects. Other LAM patients do not respond to sirolimus and continue to lose lung function quickly (17–19). Patients may not respond to sirolimus for a number of reasons, including having lung disease that is less dependent on mTOR dysregulation as a cause of tumorigenesis or recruitment of wild-type cells that support LAM cell growth and lung destruction independently of mTOR activation (13, 19, 40). For these patients, treatment with ACEIs may be an alternative. A prospective clinical trial examining the effects of ACE inhibition on LAM disease progression may be warranted.
-
Methods
Study population, ACE activity measurements, and pulmonary function testing. The retrospective study included 426 LAM patients, diagnosed by biopsy or by high-resolution CT scan with characteristic cysts and the presence of TSC, AML, LLM, elevated VEGF-D, and/or pleural effusions (9) (Table 1). Information about ACEI and sirolimus use was obtained from patients’ histories (Table 2). All medications were prescribed by the patients’ personal physicians. The sirolimus group included 102 patients receiving sirolimus, 4 receiving everolimus, and 4 who switched from one drug to the other.
Fasting serum ACE activity was measured at the NIH Clinical Center Department of Laboratory Medicine by a photometric assay (Roche Cobas e601 analyzer). Normal levels for serum ACE activity, as determined in the clinical laboratory after analysis of 28 healthy, fasting, normal volunteers, were 3–52 U/l.
Pulmonary function tests were performed (18) according to the standards of the American Thoracic Society and European Respiratory Society.
Statistics. Multiple measurements of ACE activity or pulmonary function for each patient were analyzed using mixed-effects models. These analyses were adjusted for variables such as baseline FEV1 and DLCO (from the first visit or the first visit on sirolimus), time of visit, and sirolimus treatment. Data analysis was performed with SAS 9.3. A P value less than 0.05 was considered significant.
Study approval. Protocols were approved by the National Heart, Lung, and Blood Institute IRB (protocols 95-H-0186 and 96-H-0100); written informed consent was obtained from all participants.
-
Author contributions
WKS designed the research study, acquired and analyzed data, and wrote the manuscript. MS performed the statistical analysis and wrote the manuscript. GPR and JM analyzed data and wrote the manuscript.
-
Acknowledgments
We thank Martha Vaughan for helpful discussions, and she will be missed. We also thank Angelo Taveira-DaSilva for discussions about pulmonary function testing. We thank the LAM Foundation and the Tuberous Sclerosis Alliance for their assistance in recruiting patients for our studies. This study was funded by the Intramural Research Program, National Heart, Lung, and Blood Institute, NIH.
Address correspondence to: Joel Moss, Room 6D05, Building 10, MSC 1590, National Institutes of Health, Bethesda, Maryland 20892-1590, USA. Phone: 301.496.1597; Email: mossj@nhlbi.nih.gov.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
License: Copyright 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(5):e126703. https://doi.org/10.1172/jci.insight.126703.
-
References
- Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–1228.View this article via: PubMed Google Scholar
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86(3):747–803.
- Kaparianos A, Argyropoulou E. Local renin-angiotensin II systems, angiotensin-converting enzyme and its homologue ACE2: their potential role in the pathogenesis of chronic obstructive pulmonary diseases, pulmonary hypertension and acute respiratory distress syndrome. Curr Med Chem. 2011;18(23):3506–3515.
- George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10(11):745–759.View this article via: CrossRef Google Scholar
- Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. 2005;16(7):293–299.
- Lever AF, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352(9123):179–184.
- Sun H, Li T, Zhuang R, Cai W, Zheng Y. Do renin-angiotensin system inhibitors influence the recurrence, metastasis, and survival in cancer patients?: Evidence from a meta-analysis including 55 studies. Medicine (Baltimore). 2017;96(13):e6394.
- Tan WSD, Liao W, Zhou S, Mei D, Wong WF. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol. 2018;40:9–17.
- McCormack FX, et al. Official American Thoracic Society/Japanese Respiratory Society clinical practice guidelines: Lymphangioleiomyomatosis diagnosis and management. Am J Respir Crit Care Med. 2016;194(6):748–761.
- Taveira-DaSilva AM, Pacheco-Rodriguez G, Moss J. The natural history of lymphangioleiomyomatosis: markers of severity, rate of progression and prognosis. Lymphat Res Biol. 2010;8(1):9–19.
- Matsui K, et al. Hyperplasia of type II pneumocytes in pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000;124(11):1642–1648.View this article via: PubMed Google Scholar
- Valencia JC, et al. Tissue-specific renin-angiotensin system in pulmonary lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2006;35(1):40–47.
- Clements D, Dongre A, Krymskaya VP, Johnson SR. Wild type mesenchymal cells contribute to the lung pathology of lymphangioleiomyomatosis. PLoS One. 2015;10(5):e0126025.
- Glasgow CG, et al. Involvement of lymphatics in lymphangioleiomyomatosis. Lymphat Res Biol. 2009;7(4):221–228.
- Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97(11):6085–6090.
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293.
- McCormack FX, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606.
- Taveira-DaSilva AM, Hathaway O, Stylianou M, Moss J. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann Intern Med. 2011;154(12):797–292-3.
- Bee J, Fuller S, Miller S, Johnson SR. Lung function response and side effects to rapamycin for lymphangioleiomyomatosis: a prospective national cohort study. Thorax. 2018;73(4):369–375.
- Yao J, Taveira-DaSilva AM, Jones AM, Julien-Williams P, Stylianou M, Moss J. Sustained effects of sirolimus on lung function and cystic lung lesions in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2014;190(11):1273–1282.
- Chang WY, Cane JL, Blakey JD, Kumaran M, Pointon KS, Johnson SR. Clinical utility of diagnostic guidelines and putative biomarkers in lymphangioleiomyomatosis. Respir Res. 2012;13:34. View this article via: PubMed Google Scholar
- Inoue Y, et al. Serum angiotensin-converting enzyme activity in lymphangioleiomyomatosis. Eur Respir J. 2015;46(suppl_59):PA3814.
- Karnik SS, et al. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli [corrected]. Pharmacol Rev. 2015;67(4):754–819.
- Imai N, et al. Roles for host and tumor angiotensin II type 1 receptor in tumor growth and tumor-associated angiogenesis. Lab Invest. 2007;87(2):189–198.
- Glasgow CG, Avila NA, Lin JP, Stylianou MP, Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest. 2009;135(5):1293–1300.
- Wang L, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers on lymphangiogenesis of gastric cancer in a nude mouse model. Chin Med J. 2008;121(21):2167–2171.
- Issaka RB, et al. Vascular endothelial growth factors C and D induces proliferation of lymphangioleiomyomatosis cells through autocrine crosstalk with endothelium. Am J Pathol. 2009;175(4):1410–1420.
- Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci Transl Med. 2017;9(410):eaan5616.
- Li C, et al. Estradiol and mTORC2 cooperate to enhance prostaglandin biosynthesis and tumorigenesis in TSC2-deficient LAM cells. J Exp Med. 2014;211(1):15–28.
- Pacheco-Rodriguez G, et al. Chemokine-enhanced chemotaxis of lymphangioleiomyomatosis cells with mutations in the tumor suppressor TSC2 gene. J Immunol. 2009;182(3):1270–1277.
- Podowski M, et al. Angiotensin receptor blockade attenuates cigarette smoke-induced lung injury and rescues lung architecture in mice. J Clin Invest. 2012;122(1):229–240.
- Evans SE, Colby TV, Ryu JH, Limper AH. Transforming growth factor-beta 1 and extracellular matrix-associated fibronectin expression in pulmonary lymphangioleiomyomatosis. Chest. 2004;125(3):1063–1070.
- El-Hashemite N, Kwiatkowski DJ. Interferon-gamma-Jak-Stat signaling in pulmonary lymphangioleiomyomatosis and renal angiomyolipoma: a potential therapeutic target. Am J Respir Cell Mol Biol. 2005;33(3):227–230.
- Goncharova EA, et al. Signal transducer and activator of transcription 3 is required for abnormal proliferation and survival of TSC2-deficient cells: relevance to pulmonary lymphangioleiomyomatosis. Mol Pharmacol. 2009;76(4):766–777.
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol, Cell Physiol. 2007;292(1):C82–C97.
- de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: how its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am J Physiol Heart Circ Physiol. 2015;309(1):H15–H44.
- Lee PS, et al. Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells. Am J Respir Cell Mol Biol. 2010;42(2):227–234.
- Jin Y, Han HC, Lindsey ML. ACE inhibitors to block MMP-9 activity: new functions for old inhibitors. J Mol Cell Cardiol. 2007;43(6):664–666.
- Yamamoto D, et al. Matrix metalloproteinase-2 inhibition by temocapril and its important role in peritoneal transport. Clin Exp Pharmacol Physiol. 2012;39(10):864–868.
- Dongre A, Clements D, Fisher AJ, Johnson SR. Cathepsin K in lymphangioleiomyomatosis: LAM cell-fibroblast interactions enhance protease activity by extracellular acidification. Am J Pathol. 2017;187(8):1750–1762.
- Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–1228.
-
Version history
- Version 1 (March 7, 2019): Electronic publication