Dermatology
Abstract
Fibroblast to myofibroblast transition is a critical event required for effective tissue repair. In pathologic wound repair processes, such as type 2 diabetes (T2D), fibroblast to myofibroblast transition is impaired. The exact factors that control this transition in wounds are unclear. Here, using human tissue and murine transgenic models, we show that the histone methyltransferase SETDB2 is elevated in diabetic wound fibroblasts and TNF-α represses fibroblast to myofibroblast transition via Setdb2. We identified that TNF-α increases Setdb2 in fibroblasts via a JAK1,3/STAT3 signaling pathway, where pharmacologic or genetic manipulation of this pathway altered Setdb2 in fibroblasts. We also found that fibroblasts treated with pro-inflammatory macrophage supernatants displayed increased Setdb2 and downregulated myofibroblast genes; inhibition of the TNF-α receptor reduced the upregulation of Setdb2. In diabetes, we showed that TNF-α signaling was increased in wound fibroblasts, which functions to increase Setdb2 expression and represses fibroblast to myofibroblast transition. Fibroblast-specific knockdown of SETDB2 and therapeutic inhibition of JAK1,3/STAT3 improved diabetic wound repair, where wound fibroblasts expressed increased myofibroblast genes. This study is the first to our knowledge to identify an epigenetic mechanism for reduced fibroblast to myofibroblast transition in diabetic wounds. Therapeutic targeting of the TNF-α/STAT3/SETDB2 axis in wound fibroblasts may improve diabetic wound healing.
Authors
Tyler M. Bauer, Kevin D. Mangum, Samuel D. Buckley, James Shadiow, Amrita D. Joshi, Christopher O. Audu, Jadie Y. Moon, Lindsey D. Hughes, Rachel Bogel, Lam C. Tsoi, Qinmennge Li, He Zhang, Steven Kunkel, Johann E. Gudjonsson, Frank M. Davis, Katherine A. Gallagher
Abstract
Authors
Zhehao Tan, Gio Wu, Daniela Salgado Figueroa, Paramita Dutta, Zachary Jaeger, Marissa Mazurie, David Schairer, Dawn Eichenfield, Wynnis L. Tom, Lauren Galli, Lawrence Eichenfield, Bob Geng, Brian Hinds, Hal M. Hoffman, Lori Broderick, Ben Croker, Ferhat Ay, Reid Oldenburg
Abstract
Patients with cutaneous T cell lymphoma (CTCL) experience high morbidity and mortality due to S. aureus skin infections and sepsis, but the underlying mechanisms remain unclear. We have previously identified high levels of LAIR2, a decoy protein for the inhibitory receptor LAIR1, in advanced CTCL. Mice lack a LAIR2 homolog, so we used Lair1 knock-out (KO) mice to model LAIR2 overexpression. In a model of S. aureus skin infection, Lair1 KO mice had significantly larger abscesses and areas of dermonecrosis compared to WT despite similar bacterial burdens. Lair1 KO exhibited a pattern of increased inflammatory responses in infection and sterile immune stimulation, with increased production of proinflammatory cytokines and myeloid chemokines, neutrophil ROS, and collagen/ECM pathway proteins, including collagens and complement factors. These findings support the notion that loss of LAIR1 signaling causes an excessive inflammatory response that exacerbates tissue damage and does not improve infection control. Underscoring the clinical relevance of our findings, CTCL skin lesions exhibited similarly increased expression in cytokine and collagen/ECM remodeling pathways, suggesting that high levels of LAIR2 promote excessive inflammatory tissue damage and compromise host defense against S. aureus infection. LAIR signaling represents a promising target for therapeutic development in CTCL and other inflammatory diseases.
Authors
Hannah K. Dorando, Evan C. Mutic, Kelly L. Tomaszewski, Yulia Korshunova, Ling Tian, Mellisa K. Stefanov, Chaz C. Quinn, Deborah J. Veis, Juliane Bubeck Wardenburg, Amy C. Musiek, Neha Mehta-Shah, Jacqueline E. Payton
Abstract
Mammalian skin wounds typically heal with a scar, characterized by fibrotic tissue that disrupts original tissue architecture and function. Therapies that limit fibrosis and promote regenerative healing remain a major unmet clinical need. Rosemary extract, particularly in the form of topical oils and creams, has gained widespread public attention for its purported wound-healing properties. However, its efficacy and mechanism of action remain poorly understood. We show in adult wound healing mouse models that an ethanol-based rosemary extract accelerates the speed of wound healing and mitigates fibrosis. Mechanistically, we identify that carnosic acid, a major bioactive component of rosemary leaves, activates the TRPA1 nociceptor on cutaneous sensory neurons to enhance tissue regeneration. Mice lacking TRPA1 in sensory neurons do not exhibit these pro-regenerative responses, confirming its role as a critical mediator. Together, these findings suggest that topical rosemary extract may represent an effective and accessible therapeutic approach to improve skin repair outcomes.
Authors
Emmanuel Rapp, Jiayi Pang, Borna Saeednia, Stephen Marsh Prouty, Christopher A. Reilly, Thomas H. Leung
Abstract
Impaired wound healing poses a major and increasingly frequent health problem. Among the key players in the healing process are fibroblasts, but their metabolic profile in healing wounds is largely unknown. Using a combination of transcriptomics, targeted proteomics and metabolomics, we identified retinol metabolism as a top regulated pathway in wound fibroblasts. This is functionally relevant, since even a mild retinol deficiency caused a delay in wound closure and re-epithelialization, which mainly resulted from misdirected keratinocyte migration on the new granulation tissue. Quantitative proteomics identified integrin alpha 11 (Itga11) as a less abundant protein in wounds of mice subjected to a retinol-deficient diet. Reduced levels of this fibroblast-specific protein likely altered the granulation tissue matrix, which in turn affected re-epithelialization. These results provide a comprehensive overview on the transcriptome, proteome and metabolome of wound fibroblasts and identify retinol metabolism in fibroblasts as a key regulator of tissue repair.
Authors
Till Wüstemann, Elizabeta Madzharova, Mateusz S. Wietecha, Norbert B. Ghyselinck, Marcus Höring, Gerhard Liebisch, Nicola Zamboni, Ulrich auf dem Keller, Sabine Werner
Abstract
Impairment of desmosomal cell-cell adhesion leads to life-threatening diseases such as the autoimmune skin blistering disorder pemphigus vulgaris (PV). Disease management strategies that stabilize intercellular adhesion, in addition to the existing immunosuppression therapies, may result in improved clinical outcomes. Previous findings showed that the serine protease inhibitor SERPINB5 promotes intercellular adhesion by binding to and regulating the localization of the desmosomal adapter molecule desmoplakin (DSP) at the plasma membrane. We here show that SERPINB5 overexpression prevents PV-IgG-mediated loss of cell-cell adhesion and DSP dissociation from the cell membrane. We mechanistically demonstrate that SERPINB5 loss deregulates TGF-β signalling, a pathway known to destabilize DSP in keratinocytes. TGF-β signalling was also activated in skin biopsies of PV patients and keratinocytes treated with PV autoantibodies, suggesting a contribution to disease. Inhibition of TGF-β signaling ameliorated PV-IgG-mediated loss of cell-cell adhesion, increased DSP membrane expression, and prevented PV-IgG-induced blister formation in a human ex-vivo skin model. Together, SERPINB5 modulates DSP and intercellular adhesion through the regulation of TGF-β signalling. Further, TGF-β signalling was identified as a potential target for pemphigus treatment.
Authors
Maitreyi Rathod, Mariam Petrosyan, Aude Zimmermann, Maike Märker, Tobias Gosau, Henriette Franz, Tomás Cunha, Dario Didona, Michael Hertl, Enno Schmidt, Volker Spindler
Abstract
Mucous membrane pemphigoid (MMP) is a mucocutaneous autoimmune blistering disease affecting diverse mucous membranes and the skin with inflammatory blisters and erosions. The pathogenesis of MMP is only poorly understood, but inflammation in MMP is triggered by specific binding of autoantibodies directed to different proteins of the dermal-epidermal/-epithelial junction, subsequently leading to the influx of inflammatory cells, particularly neutrophils, into the dermis. Using the anti-laminin 332 antibody transfer model of MMP, we addressed the molecular mechanisms of neutrophil infiltration and its significance for the eruption of mucocutaneous lesions. Mice deficient in 5-lipoxygenase (Alox5–/–) or in the leukotriene B4 (LTB4) receptor BLT1 (Ltb4r1–/–) were resistant to skin inflammation and exhibited substantially fewer mucosal lesions, with deficiency in either gene compromising the recruitment of neutrophils to the lesion. Furthermore, neutrophil-specific genetic deficiency in Ltb4r1 similarly protected from MMP. Hence, BLT1 was required on neutrophils, and neutrophil recruitment was indispensable for the eruption of lesions in MMP. In line with these findings, the BLT1 inhibitor CP-105,606 ameliorated MMP dose-dependently. Collectively, our results highlight neutrophils and LTB4/BLT1 as key drivers of inflammation in MMP and as promising therapeutic targets.
Authors
Tabea Bremer, Sripriya Murthy, Sabrina Patzelt, Paul Schilf, Mareike Neumann, Sina Gonther, Jasper Pruessmann, Wiebke Pruessmann, Enno Schmidt, Thomas Rülicke, Christian D. Sadik
Abstract
Palmoplantar pustulosis (PPP) is a chronic inflammatory skin disorder marked by erythematous pustules and desquamation on the palms and soles. While IL-17 pathways are implicated in PPP, IL-17 blockers have shown modest efficacy, underscoring the need for a deeper understanding of IL-17 involvement. To dissect the cellular and spatial architecture of PPP, we performed single-cell RNA-Seq (scRNA-Seq) on lesional, nonlesional, and healthy acral skin to examine cellular composition, transcriptomic profiles, and cell-cell interactions. Unbiased clustering revealed 9 major cell types, including an inflammatory keratinocyte subset enriched in IL-17A/TNF signatures and marked by high IL-36G expression. Within the lymphocyte compartment, we identified a hybrid “regTh17” population coexpressing regulatory markers (FOXP3, CTLA4, TIGIT), IL17F, and IL26. This regTh17 subset was distinguished by elevated IL1R1 and CD39, suggesting an IL-1β–driven differentiation. Spatial analyses demonstrated significant neighborhood enrichment of regTh17 cells with IL-36G+ supraspinous keratinocytes. RegTh17 cells were the predominant source of IL-17F and IL-26 signals, whereas keratinocytes were predicted as their main receivers. We further observed regTh17 coexpressing TNFRSF4 (OX40) and TNFRSF18 (GITR) specifically at sites of IL36G+ keratinocyte interactions, implicating these pathways in amplification of the IL-17/IL-36 inflammatory loop. Together, our integrated single-cell and spatial profiling uncovers Th17 plasticity in PPP, identifies a regTh17-keratinocyte interaction, and highlights IL-17F, IL-26, OX40/OX40L, and GITR/GITRL as candidate targets for precision therapies in this challenging disease.
Authors
Tran H. Do, Rachael Bogle, Haihan Zhang, Xianying Xing, Mehrnaz Gharaee-Kermani, Madalina Raducu, Jennifer Fox, Rundong Jiang, Olesya Plazyo, Paul W. Harms, Mio Nakamura, Enze Xing, Michel Gilliet, Allison C. Billi, J. Michelle Kahlenberg, Robert L. Modlin, Ozge Uluckan, Lam C. Tsoi, Johann E. Gudjonsson
Abstract
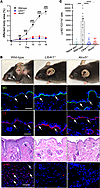
Secondary lymphedema is characterized by fibrosis and impaired lymphatic function. Although TGF-β is a key regulator of fibrosis in this disease, the cellular mechanisms regulating this process remain unknown. Epithelial–mesenchymal transition (EMT), a mechanism by which TGF-β induces fibrosis in other skin diseases, is characterized by loss of epithelial cell markers and cellular polarity, upregulation of fibrotic gene expression, and gain of migratory capacity. Using clinical lymphedema biopsy specimens and animal models, we show that keratinocytes in the basal layer of the epidermis undergo EMT in lymphedematous skin, migrate into the dermis, and contribute to dermal fibrosis. In vitro studies using cultured primary human keratinocytes treated with lymphatic fluid from the affected limbs of patients with secondary lymphedema resulted in a TGF-β–mediated increased expression of EMT markers. We show for the first time that EMT is activated by TGF-β in secondary lymphedema and that this process plays an important role in regulating skin fibrosis in this disease.
Authors
Hyeung Ju Park, Jinyeon Shin, Ananta Sarker, Mark G. Klang, Elyn Riedel, Michelle Coriddi, Joseph H. Dayan, Sarit Pal, Babak J. Mehrara, Raghu P. Kataru
Abstract
Psoriasis is a chronic autoimmune skin disease characterized by abnormal keratinocyte proliferation and immune dysregulation. Altered lipid metabolism has been implicated in its pathogenesis, but the underlying mechanisms remain unclear. In this study, we generated an keratinocyte-specific Sprouty RTK signaling antagonist 1 (SPRY1) knockout (Spry1ΔEpi) mouse model, which exhibits psoriasis-like symptoms. Using both psoriasis patient samples and Spry1ΔEpi mice, we investigated the role of diacylglycerol acyltransferase 2 (DGAT2) in psoriasis. Our results show that DGAT2 expression is reduced, and glycerides metabolism is disrupted in psoriatic lesions in both psoriasis patients and Spry1ΔEpi mice. Lipidomic analysis reveals significant alterations in glycerides, glycerophospholipids, sphingolipids, and fatty acids in Spry1ΔEpi mice. At the cellular level, DGAT2 downregulation and lipid dysregulation enhance Toll-like receptor 3 (TLR3)-mediated inflammatory signaling in keratinocytes. Furthermore, increased DGAT2 secretion from keratinocytes promotes CD8⁺ T cell activation, proliferation and survival, amplifying psoriatic inflammation. These findings highlight the role of DGAT2 and lipid metabolism in the pathogenesis of psoriasis and reveal their interaction with immune responses in psoriasis.
Authors
Ying-Ying Li, Li-Ran Ye, Ying-Zhe Cui, Fan Xu, Xi-Bei Chen, Feng-Fei Zhang, Yi Lu, Yu-Xin Zheng, Xiao-Yong Man
No posts were found with this tag.