Issue published February 9, 2026
- Volume 11, Issue 3
- Previous Issue
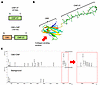
Ogmen et al. report clinical features and disease mechanisms in patients with microcephaly-lymphedema-chorioretinopathy with KIF11 pathogenic variants, focusing on lymphatic function. The cover image shows confocal immunofluorescence microscopy of a paraffin section of human duodenum from a patient with a KIF11 variant. E-cadherin labels epithelial cells in yellow, PDPN marks lymphatic vessels in magenta, CD31 visualizes blood vessels in cyan, and nuclei are shown in orange. Image credit: Pia Ostergaard, Kazim Ogmen, Silvia Martin-Almedina, Felix Heymann, Rose Yinghan Behncke, and René Hägerling.
On the cover: Insights into KIF11 pathogenesis in microcephaly-lymphedema-chorioretinopathy syndrome from a lymphatic perspective
In-Press Preview - More
Abstract
Hirschsprung disease (HSCR) is a congenital intestinal disorder characterized by the absence of ganglia in the distal intestine. Despite surgical resection of the aganglionic intestine and pull-through surgery, HSCR patients still experience bowel dysfunction, indicating that latent abnormalities may also exist in the proximal ganglionic intestine. To elucidate possible causes of postoperative bowel dysfunction in HSCR, we investigated differences in the proximal ganglionic intestine using an animal model of HSCR (Ednrb-null mice) and validated our findings in tissue from human HSCR patients. We found that the proximal ganglionic colon of HSCR mice exhibited greater stiffness and fibrosis than their wild-type littermates. Similarly, submucosal fibrosis was significantly greater in the proximal ganglionic intestine of HSCR patients than in intestinal tissue from age and site-matched controls. Furthermore, we observed dysregulated expression of extracellular matrix (ECM)-related genes in the proximal ganglionic intestine of HSCR mice compared to controls. We conclude that increased fibrosis, stiffness, and alterations in ECM composition may contribute to persistent dysfunction of the ganglionic intestine in HSCR. These findings add to the growing body of literature that describe abnormalities in the proximal ganglionic intestine of HSCR and suggest that HSCR is not limited to the aganglionic intestine alone.
Authors
Prisca C. Obidike, Britney A. Hsu, Chioma Moneme, Oluyinka O. Olutoye II, Walker D. Short, Mary Hui Li, Swathi Balaji, Yuwen Zhang, Sundeep G. Keswani, Lily S. Cheng
Abstract
Dysfunctional white adipose tissue contributes to the development of obesity-related morbidities, including insulin resistance, dyslipidemia, and other metabolic disorders. Adipose tissue macrophages (ATMs) accumulate in obesity and play both beneficial and harmful roles in the maintenance of adipose tissue homeostasis and function. Despite their importance, the molecules and mechanisms that regulate these diverse functions are not well understood. Lipid-associated macrophages (LAMs), the dominant subset of obesity-associated ATMs, accumulate in crown-like structures and are characterized by a metabolically activated and proinflammatory phenotype. We previously identified CD9 as a surface marker of LAMs. However, the contribution of CD9 to the activation and function of LAMs during obesity is unknown. Using a myeloid-specific CD9 knockout model, we show that CD9 supports ATM-adipocyte adhesion and crown-like structure formation. Furthermore, CD9 promotes the expression of pro-fibrotic and extracellular matrix remodeling genes. Loss of myeloid CD9 reduces adipose tissue fibrosis, increases visceral adipose tissue accumulation, and improves global metabolic outcomes during diet-induced obesity. These results identify CD9 as a causal regulator of pathogenic LAM functions, highlighting CD9 as a potential therapeutic target for treating obesity-associated metabolic disease.
Authors
Julia Chini, Nicole DeMarco, Dana V. Mitchell, Sam J. McCright, Kaitlyn M. Shen, Divyansi Pandey, Rachel L. Clement, Jessica Miller, Rajan Jain, Deanne M. Taylor, Mitchell A. Lazar, David A. Hill
Abstract
Latently infected cells persist in people living with HIV (PWH) despite suppressive antiretroviral therapy (ART) and evade immune clearance. Shock and Kill cure strategies are hampered by insufficient enhancement of targeted immune responses following latency reversal. We previously demonstrated autologous Vδ2 T cells from PWH retain anti-HIV activity and can reduce CD4+ T cell reservoirs, although their use in cure approaches is limited due to their dual role as a viral reservoir. However, promising clinical data in oncology shows their unique MHC- unrestricted antigen recognition affords potent on-target cytotoxicity in the absence of graft-versus-host disease when used as an allogeneic adoptive cell therapy modality. Here, we found expanded allogeneic Vδ2 T cells specifically eliminated HIV-infected CD4+ T cells and monocyte-derived macrophages (MDM), overcoming inherent resistance to killing by other cell types such as NK and CD8+ T cells. Notably, we demonstrated allogeneic Vδ2 T cells recognized and eliminated the HIV-latent CD4+ T cell reservoir following latency reversal. Our study provides evidence for developing an allogeneic γδ T cell therapy for HIV cure and warrants pre-clinical investigation in combination approaches.
Authors
Brendan T. Mann, Marta Sanz, Alisha Chitrakar, Kayley Langlands, Marc Siegel, Natalia Soriano-Sarabia
Abstract
Infectious diseases remain a global health challenge, driven by increasing antimicrobial-resistance and the threat of emerging epidemics. Mycobacterium tuberculosis and Staphylococcus aureus are leading causes of mortality worldwide. Trained immunity—a form of innate immune memory—offers a promising approach to enhance pathogen clearance. Here, we demonstrate that IFN-γ induces trained immunity in human monocytes through a mechanism involving mTORC1 activation, glutaminolysis, and epigenetic remodeling. Macrophages derived from IFN-γ–trained monocytes exhibited increased glycolytic activity with enhanced cytokine and chemokine responses upon stimulation or infection. Crucially, trained macrophages had increased production of reactive oxygen species which mediated enhanced bactericidal activity against methicillin-resistant S. aureus. Furthermore, ATAC-sequencing analysis of IFN-γ trained macrophages revealed increased chromatin accessibility in regions associated with host defence. Lastly, IFN-γ training restored impaired innate responses in macrophages from individuals homozygous for the TIRAP 180L polymorphism, a genetic variant associated with increased susceptibility to infection. These findings establish IFN-γ as a potent inducer of trained immunity in human monocytes and support its potential as a host-directed strategy to strengthen antimicrobial defenses, particularly in genetically susceptible individuals and high-risk clinical contexts.
Authors
Dearbhla M. Murphy, Isabella Batten, Aoife O'Farrell, Simon R. Carlile, Sinead A. O'Rourke, Chloe Court, Brenda Morris, Gina Leisching, Gráinne Jameson, Sarah A. Connolly, Adam H. Dyer, John P. McGrath, Emma McNally, Olivia Sandby-Thomas, Anjali Yennemadi, Conor M. Finlay, Clíona Ni Cheallaigh, Jean Dunne, Cilian Ó Maoldomhnaigh, Laura E. Gleeson, Aisling Dunne, Nollaig Bourke, Reinout van Crevel, Donal J. Cox, Niall Conlon, Arjun Raj, Rachel M. McLoughlin, Joseph Keane, Sharee A. Basdeo
Abstract
Nicotinamide adenine dinucleotide (NAD⁺) is essential for cellular metabolism, DNA repair, and stress responses. NAD+ is synthesized from nicotinamide, nicotinic acid (collectively termed niacin), and tryptophan. In humans, deficiencies in these nutrients result in pellagra, marked by dermatitis, diarrhea, and dementia. The dermatitis associated with pellagra typically manifests as photodermatosis in sun-exposed areas. This study examined the effects of NAD+ deficiency on skin homeostasis using epidermis-specific Nampt conditional knockout (cKO) mice. These mice displayed substantial NAD⁺ depletion, reduced poly(ADP-ribose) polymerase (PARP) activity, and increased DNA damage. Consequently, Nampt cKO mice developed spontaneous skin inflammation and epidermal hyperplasia. RNA sequencing and immunohistochemical analyses demonstrated increased interleukin-36 (IL-36) cytokine expression, suggesting that DNA repair-related genomic stress triggers keratinocyte-driven IL-36 production, which promotes inflammation. Furthermore, reduced collagen17A1 expression and elevated thymic stromal lymphopoietin (TSLP) levels were observed. NAD+ repletion by transdermal supplementation of nicotinamide mononucleotide (NMN) suppressed the rise of IL-36 levels and skin inflammation. These findings underscore the importance of Nampt-mediated NAD⁺ metabolism for epidermal stability and indicate that NAD⁺ depletion may contribute to IL-36-mediated skin inflammation, offering insights for therapeutic strategies in inflammatory skin disorders.
Authors
Taiki Seki, Jun-Dal Kim, Yasuhito Yahara, Hitoshi Uchida, Keisuke Yaku, Mariam Karim, Teruhiko Makino, Tadamichi Shimizu, Takashi Nakagawa